HYDROGEN BOND NETWORK IN LIQUID WATER AND ASSESSMENT OF INDICATORS OF ITS RESTRUCTURING UNDER THE EXTERNAL INFLUENCE
Абдуллаев Арслан-Али. Абдуллаевич1, Рабаданов Гаджи. Аппасович2, Гашимова Энара. Джебраиловна3
1Ведущий научный сотрудник, 2заведующий лабораторией,3аспирантка, ФГБУН Институт проблем геотермии Дагестанского научного центра РАН
СЕТКА ВОДОРОДНЫХ СВЯЗЕЙ В ЖИДКОЙ ВОДЕ И ОЦЕНКА ПОКАЗАТЕЛЕЙ ЕЕ ПЕРЕСТРОЕК ПОД ВЛИЯНИЕМ ВНЕШНЕГО ВОЗДЕЙСТВИЯ
Аннотация
Установлен способ сохранения части энергии, выделяемой при образовании водородных связей в виде энергоемкости их сетки и ее использования при изменении конфигураций локальных ассоциаций молекул воды.
Показано, что протонная донорно-акцепторная активность молекул при выполнении условий, определенных в работе может обеспечить возникновение синхронных переходов протонов от одиночных молекул к молекулам сетки Н-связей и изменить форму молекул, входящих в состав их локального объединения (кластера).
Ключевые слова: сетка водородных связей, представительный элемент сетки, энергоемкость сетки.
Abdullaev Arslan-Ali.A.1, Rabadanov Gadji.A.2, Gashimova Enara.D.3
1Leading researcher, 2Head of laboratory, 3Postgraduate student, Institute of Geothermy Problems, Dagestan Scientific Centre, Russian Academy of Sciences
HYDROGEN BOND NETWORK IN LIQUID WATER AND ASSESSMENT OF INDICATORS OF ITS RESTRUCTURING UNDER THE EXTERNAL INFLUENCE
Abstract
The article presents the way to keep a part of energy of formation of hydrogen bonds in water structure and to use it by changing local associations of H2O molecules.
It is shown that if the conditions stated in the paper are fulfilled, proton donor-acceptor activity of molecules may provide synchronous proton transfers from single molecules to molecules of H-bond network and change the shape of the molecules forming part of their local cluster.
Keywords: network of hydrogen bonds, a representative element of the grid, the energy intensity of the grid.
Introduction
Hydrogen bond (H-bond) is of great importance in defining properties of many compounds [1].
The role of H-bonds in water has not been fully determined yet. Particularly, the certain type of molecule interaction that is responsible for formation of H-bond between molecules has not been established, as well as the conditions for formation of H-bond network and conditions and regularities of its existence.
Complexity of H-bonds description is conditioned by the fact that they cannot be described by static potential due to dissipative character of the processes of H-bond formation and breaking.
The works [2,3] show that resonance interaction of atoms of different molecules [3,4] may be assumed as the interaction responsible for H-bonding.
The present paper covers the conditions of formation of H-bond network, evaluates its role in water structuring and reveals effective properties of H-bond network.
The theoretical part
It is known [5] that during H-bond formation not all expected energy is released: part of the energy is conserved in the arising structure as configurational or resonance energy. Configurational energy helps water to change its space configuration easily. However, interaction of H-bonds between each other results in changing of configurational energy, so it is necessary to use another value instead, which will take into account not only interaction of H-bonds, but the number of free centers of proton trapping by oxygen atoms of the molecules of interacting H-bonds as well.
The papers [4, 6] show that excess energy of H-bond is defined by the value:
where ɛн is the constant of energy dimension that does not depend on the parameter a and external factors.
It was revealed long ago [2] that H-bonds interact between each other mainly in the plane of their position. Therefore, resonance influence of H-bonds between each other may be presented as
where np is the number of protons forming interacting H-bonds.
Having multiplied the summands in expression [2] and dropped the members containing the parameter a in the fourth power we get
To determine the influence of H-bond network on the observed properties of water it is necessary first to formulate the properties of H-bond network itself. One of the features of continuous H-bond network is the representational element (RE) of the network.
It is convenient to use the total of two interacting H-bonds, oxygen atoms of which have eight electrons able to catch up to four protons, as the RE. If we assume the RE as the elementary cell of continuous H-bond network, octet rule will be peculiar to both network and electron shell of atoms.
The problem of structure and role of H-bond network will be considered below and here we present the characteristics of the network.
As the required energy capacity characteristic of RE of H-bond network it is convenient to use partly proved above expression of energy capacity of RE of H-bond network:
where nв is the average number of free centers of proton trapping by oxygen atoms of the molecules of the RE of H-bond network.
We should point out that here and above the dimensionless parameter a depends on factors of external influence, and particularly, on temperature according to the law [6]:
Using the dependences (5), the expression of energy capacity of RE of H-bond network can be written in the form:
Analysis of J(T) expression shows that the degree of filling of the trapping centers of oxygen atoms of the molecules of RE depends on temperature. The study of this dependence shows [6] that it can be approximated by the formula:
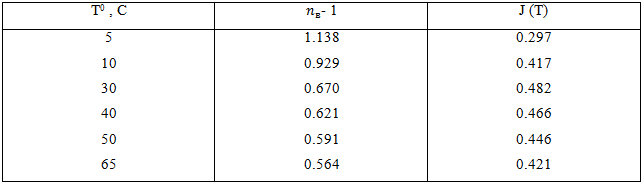
If we take into consideration the dependence (nв – 1), temperature dependence of energy capacity from T can be determined from the data of Table 1.
Table 1 - Energy capacity dependence on temperature in the absence of synchronous proton transfers
However, it ought to be noted that monotonous character of filling of oxygen centers of proton trapping can change sharply allowing for synchronous proton transfers (SPT) from single molecules to H-bond network [6,7].
It has been revealed that SPT occur at the definite temperature and on condition that excess energy of interaction of atoms of different molecules coincides with energy of interaction of deformation charges of H-bonds. It unambiguously estimates the Tc temperature of SPT fulfillment: Tc=42,68˚C [8].
As a result, the conditions for full filling of centers of proton trapping by oxygen atoms of molecules of RE of H-bond network are formed in local areas of liquid water at Tc temperature [8].
Analysis of the state of H-bond network with two bonds per molecule shows that on account of repulsion of deformation charges of H-bonds the network becomes non-stationary in regard to division into autonomous volume clusters (AVC) [9].
As the process of division of H-bond network, contrary to its creation, goes with the expenditure of additional energy it should take place at the point of change of the process of energy release into the process of energy absorption. Consequently, at the given point the following equalities must be valid:
Using the conditions (8) in the equality (6), we find
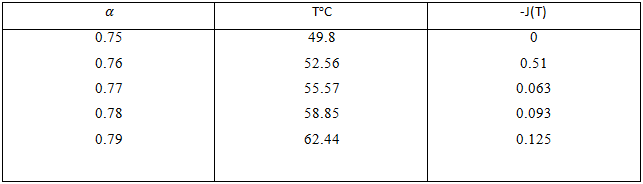
On account of division of H-bond network into AVC, the existence of H-bond network in liquid water suggests the processes of molecule coupling and decoupling in it. As the main indicator of H-bond network restructuring in the scheme under consideration is energy capacity J(T), it is necessary to give some data on J(T) without and with the participation of SPT for cases nв=1 and nв=0.
Table 2 - Dependence of energy capacity of representational element of the network with hydrogen- unsaturated molecules
According to Table 2, the growth of temperature causes decrease of energy capacity, which is monotonous, unlike the case without SPT.
Table 3 - Dependence of energy capacity of autonomous volume cluster with hydrogen- saturated molecules on temperature
Dependence of energy capacity also decreases with the growth of temperature. J(T) values for AVC are less than for RE of the network and they differ in sign.
During division of H-bond network the smallness of absorption energy as compared to configurational energy of H-bond is quite expected. It is conditioned by two factors: firstly, during cell division a part of stored energy keeps in the forming structure, i.e. in AVC, and secondly, AVC does not dissipate due to hydrogen-saturation of its molecules.
Conclusion (discussion of results)
In the paper unshared electron pairs in the oxygen atom of H2O molecule are regarded as centers of proton trapping. As a rule, transfer of two protons to two unshared electron pairs of oxygen atom of molecule results in intense deformation of its electron shell. Particularly, electron pairs in the shell of oxygen atom may disorder under the proton influence. It is important to stress that the most profound changes including the molar structure are conditioned by the participation of synchronous proton transfers in the process.
Physically interesting property of water with hydrogen- saturated molecules is the absence of donor-acceptor activity and dissipation in them.
Another important change of water structure at molecular level is connected with the possibility to constitute conditions for formation of H2 hydrogen molecule in the electron shell of oxygen atom with two trapped protons.
Therefore, when the foregoing conception is correct, H-bond network may be regarded as the frame of oxygen atoms with hydrogen shell. Hydride molecule is known to be double acceptor of protons.
However, on account of low donor-acceptor activity of hydride molecules, except water, not all centers of proton trapping in them appeared to be energetically accessible.
In water the structure with hydrogen-saturated molecules appear to be accessible with high probability due to high donor-acceptor activity of H2O molecules and presence of SPT.
References
- Chang, Physical Chemistry with Supplements to Biological Systems. Mir Publishing House, Moscow, 1980. 662 p.
- Tsyan Syuz-sen, Physical Mechanics. Mir Publishing House, Moscow, 1965. 544 p.
- Davydov A.S. Quantum Mechanics. Publishing House of Physico-Mathematical Literature, Moscow, 1963, 744 p.
- Abdullaev A.A. Symmetries of Quantum of Oscillatory Systems. Nuclear Physics Journal. 1968, No.6, p. 1339.
- Poltorak O.M. and Kovba L.M. Physico-Chemical Fundamentals of Inorganic Chemistry. Publishing House of Moscow University. 1984, 287 p.
- Abdullaev A.A. Network of Hydrogen Bonds and Criteria of Their Division in Liquid Water. Natural and Engineering Sciences Journal. Moscow, 2013, No. 3, pp. 30-31.
- Abdullaev A.A. and Rabadanov G.A. Study of Factors of External Influence on Water through Hydrogen Bonds and Assessment of its Efficiency. Proceedings of International Research/Practice Conference. August 30, 2014. Part 4. Tambov, 2014.
- Abdullaev A.A. and Rabadanov G.A. Unusual Processes and Phenomena in Water and Their Physical Substantiation. Italian Science Review Journal. October 10, 2014. ias-jornah.org.
- Abdullaev A.A. and Gasanaliev A.M. Electric and Magnetic Properties of Local Associations of Molecules in Liquid Water. Proceedings of Dagestan State Pedagogical University. Natural and Exact Sciences. 2014, No. 3, pp.6-9.