PRENATAL DEXAMETHASONE TREATMENT: LONG-TERM COGNITIVE OUTCOME IN CHILDREN
Шайтарова А.В.¹, Суплотова Л.А.², Храмова Е.Б.³
¹кандидат медицинских наук, ²доктор медицинских наук, профессор, ³доктор медицинских наук, доцент, Тюменский государственный медицинский университет
ПРЕНАТАЛЬНАЯ ТЕРАПИЯ ДЕКСАМЕТАЗОНОМ: ОТСРОЧЕННЫЕ ЭФФЕКТЫ НА ИНТЕЛЕКТУАЛЬНОЕ РАЗВИТИЕ ДЕТЕЙ
Аннотация
Врожденная дисфункция коры надпочечников, обусловленная дефицитом 21-гидроксилазы, является распространенным, потенциально летальным заболеванием. Его частота, рассчитанная по данным неонатального скрининга, составляет 1 случай на 14000 живых новорожденных в мировой популяции, 1 на 9500 - в России [1]. В течение двух десятилетий в большинстве стран мира проводится пренатальная терапия дексаметазоном с целью предотвращения вирилизации наружных гениталий и влияния избытка андрогенов на половую дифференцировку мозга у ХХ-плода с дефицитом 21-гидроксилазы. Однако дексаметазон эффективен у плодов женского пола с ВДКН при условии, что терапия инициирована до 6-ой недели гестации [2]. Дефицит 21-гидроксилазы является аутосомно-рециссивным заболеванием, и в семье, имеющей больного ребенка, вероятность беременности больной девочкой составляет 12,5%. Поскольку пренатальная диагностика возможна только на 10 неделе беременности, то в 87,5% случаев плод будет получать высокие дозы глюкокортикоидов напрасно с 4-6 до 10 недели гестации. Пренатальная терапия не позволяет избежать в дальнейшем пожизненной терапии и не является профилактикой сольтеряющих состояний в послеродовом периоде, а безопасность дексаметазона, относительно познавательных способностей детей, получавших его в период внутриутробного развития, остается спорным вопросом.
Ключевые слова: глюкокортикоиды, беременность, отсроченные эффекты, поведение, дети
Shaytarova A.V.¹, Suplotova L.A.², Khramova E.B.³
¹MD, ²MD, professor,³MD, associate professor, Tyumen State Medicine University
PRENATAL DEXAMETHASONE TREATMENT: LONG-TERM COGNITIVE OUTCOME IN CHILDREN
Abstract
The andrenogenital syndrome responding to 21-hydroxylase deficiency is a common, potential fatal disease. Its incidence calculated on the basis of neonatal screening data makes 1 case for 14000 live newborns among the worldwide population , 1 for 9500 – in Russia [1]. During the last twenty years in most countries prenatal DEX-treatment has been used to prevent genital virilisation and androgen excess outcome on sex brain differentiation of XX-foetus with 21-hydroxylase deficiency. However, dexamethasone is considered to be effective in treating CAH-female foetus subject to the condition that the treatment is initiated before 6th gestation week [2]. 21-hydroxylase deficiency is an autosomal recessive disease and the probability of diseased female pregnancy makes 12.5% in a family already having a child with such disease. Considering the fact that prenatal diagnostics is only possible at 10th gestation week, in 87.5% of cases a foetus will be exposed to high doses of glucocorticoids without any effect from 4-6th up to 10th gestation weeks. The prenatal treatment does not preclude from a life-long treatment in future and it is not prevention of a salt-losing syndrome at the postnatal period, and dexamethasone safety in relation to cognitive development of children prenatally treated with dexamethasone is still up for debate.
Key words: glucocorticoids, pregnancy, long-term outcome, behavior, childrenOBJECTIVES:
to study possible long-term effects of prenatal glucocorticoid treatment on children cognition.
DESIGN:
the prospective research of intellectual development patterns of 288 children from mothers, prenatally treated with dexamethasone, and of 107 children (the observational group) from mothers, not treated with dexamethasone, with high biochemical markers of adrenal hyperadnrogenism.
METHODS:
from 2002 to 2011 the children of the treatment group (n=288) were examined at the age of 1 (n=144), at the age of 2 (n=58), at the age of 3 (n=26) and up to the primary school age (mean 7, 8 years, rang 7-9) – (n=90). The retrospective review of prenatal records showed that the average daily dose of prenatal dexamethasone was 2,44±1,17 μ/kg of body mass and the duration of glucocorticoid treatment varied from 6 to 24 weeks (22,8±1,6 weeks). 57 infants of early childhood and 50 children of primary school age were included into the observational group. The early age children neuropsychic development was assessed via average development index assay (ИРср) for every psychoneurological function according the methods of I.A. Skvortsova, N.A. Ermolenko. The intellectual development patterns of primary school age children were investigated using a children form of WAIS test.
The present clinical research has been approved by Ethics Committee and conducted pursuant to the Declaration of Helsinki. All parents/guardians of the patients included into the research have signed patient informed consents.
RESULTS:
examining children of the similar groups at an earlier age (1-3 years old) has not revealed any differences of their intellectual development (р < 0,5). The level of general intelligence of children whose mothers have been treated with dexamethasone in I and II trimesters of pregnancy is considerably lower than that of children from the observational group. These children have been also characterized with the impairment of speech development (р = 0,005), verbal reasoning and logical thinking (p = 0,0003), maturity of volitional attention and ability of organizing and monitoring activities (р=0,01).
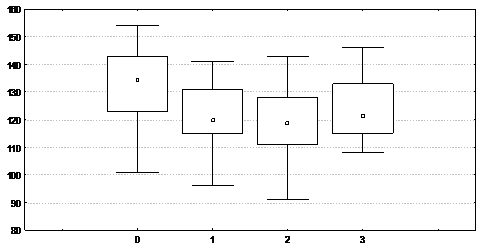
Fig. 1. School Age Children’s IQ Quotient of Treatment and Observational Groups
(0- Observational Group, 1- DEX-treatment in I trimester, 2- DEX-treatment in II trimester, 3- DEX-treatment in III trimester)
No differences of cognitive development between children, prenatally treated with dexamethasone in III trimester, and the observational group have been observed.
CONCLUSIONS:prenatal DEX-treatment at an early gestation can result in significant adverse effects on intellectual abilities of children furtheron.
References
- Kareva M.A.; Chugunov I.S. Federal clinical practice guidelines on the management of the patients presenting with congenital adrenal hyperplasia// Problemy Endokrinologii, v.60, n2, p.42-50, april 2014. ISSN 0375-9660 (Print) 2308-1430 (Electronic). doi: 14341/probl201460242-50.
- Tardy-Guidollet V, Menassa R, et all. New management strategy of pregnancies at risk of congenital adrenal hyperplasia using fetal sex determination in maternal serum: French cohort of 258 cases (2002-2011)// . Epub. - 2014 Apr; 99 (4):1180-8. doi: 10.1210/jc.2013-2895
