Investigation of the plasma composition of hollow cathode arc discharge with active Al anode in hexamethyldisilazane/tetraethoxysilane vapors
Investigation of the plasma composition of hollow cathode arc discharge with active Al anode in hexamethyldisilazane/tetraethoxysilane vapors
Abstract
Optical emission spectroscopy was used to analyze the plasma composition during reactive evaporation of Al in an arc discharge with a self-heating hollow cathode (SHHC) in a gas mixture of Ar+N2/Ar+O2 in vapors of organosilicon compounds (OSC) hexamethyldisilazane (HMDS) and tetraethoxysilane (TEOS) at a pressure of ~10-4 Torr. The density of the total ion saturation current per sample, with a fraction of metal ions up to 50%, reached 10 mA/cm2, which is an important condition for the formation of high-quality dense coatings. The influence of fluxes of organosilicon precursors, reactive gases, the current of the main discharge gap and the flow of Al vapors on the degree of decomposition of HMDS and TEOS and on the plasma composition has been studied. High rates of decomposition of the initial HMDS and TEOS molecules were achieved, as well as high values of the degree of dissociation of N2 (up to 0,07) and O2 (up to 0,4). Trial coatings of SiAlCN and SiAlCO with a homogeneous structure and good adhesion to stainless steel substrates were obtained. The hardness of the SiAlCN test films reached 30 GPa, and the hardness of SiAlCO reached 12 GPa. Thus, it is shown that this method of activation of a vapor-gas mixture provides an intensive flow and a high degree of ionization of Al vapors, and also allows for deep decomposition of organosilicon precursors of HMDS and TEOS to form coatings of the desired composition, good quality with high deposition rates.
1. Introduction
The development of ion-plasma methods for the synthesis of films and coatings for various purposes is one of the relevant areas of research in the field of surface modification and the production of new materials. Currently, methods based on the plasma decomposition of organosilicon compounds (OSC) are being developed for the deposition of multicomponent films with unique properties. The use of liquid organosilicon precursors is due to one of the most affordable methods of obtaining silicon in the gas phase, their low cost, as well as a wide range of precursors with different chemical compositions and the content of elements Si, C, N, O etc. At the same time, the advantage of using OSC to obtain multicomponent films is also the presence of Si-C, Si-N, etc. bonds in the molecule, the incomplete decomposition of which upon activation in plasma facilitates the formation of the desired chemical structure of coatings. As a rule, films obtained from such precursors have the same elemental ratio as in the initial precursor molecules . The elemental composition of the synthesized films can be adjusted by adding reactive components to the gaseous medium, for example, gases CH4, N2 and O2, which significantly corrects the chemical composition of the coatings obtained and, accordingly, their properties. In addition, a metal component can also be added to such a combined-gas mixture and multicomponent metal-ceramic coatings with a unique combination of properties can be obtained. For example, one of such promising materials are compounds based on SiAlCN and SiAlCO, which have replaced ceramic materials based on SiCN and SiCO. As technologies have improved, for example, in the nuclear power industry or the gas turbine industry, the task has become to develop more efficient materials capable of operating under extreme conditions with increased loads. SiAlCN and SiAlCO have a unique set of properties. SiAlCO has a high coefficient of piezo sensitivity (strain sensitivity), so they can be used to create smaller sensor systems. SiAlCN have abnormal oxidation resistance at temperatures up to 1400°C due to the addition of Al . In addition, such coatings have high temperature resistance , high hardness and wear resistance . Therefore, such coatings are ideal for protecting components that are subject to wear at elevated temperatures, as well as for creating sensitive, compact sensors for extreme applications.
A promising method for the synthesis of such coatings is the hybrid plasma-chemical PVD+PECVD method , based on the evaporation of metal by a stream of electrons from an arc charge with a hollow cathode in a medium containing vapors of organosilicon precursors. The advantage of this method is the ability to change almost all deposition conditions in a wide range to select the optimal synthesis mode to obtain the desired coating properties. Also, the use of a gas discharge can provide an intense ion flux necessary for the formation of a dense qualitative coating structure , and low-temperature plasma decomposition of precursors, in contrast to thermal activation carried out at temperatures of ~1000°C, will allow the synthesis of coatings on a wider range of materials.
The purpose of this work is to study the conditions for the formation of an active vapor-gas medium by the method of anodic evaporation of Al in an arc discharge plasma with a self-heating hollow cathode (SHHC) in an Ar+N2/Ar+O2 medium containing pairs of organosilicon compounds HMDS/TEOS for the deposition of promising PDC coatings based on SiAlCN and SiAlCO. The paper presents the results of a study of the effect of various discharge parameters with a self-heating hollow cathode and a sectional anode, including the discharge current, the type of precursor, the amount of precursor consumption and reactive components of the gaseous medium, on the plasma composition, including the degree of dissociation of various plasma components and its ionic composition.
2. Experimental methods
Experimental studies of plasma-chemical activation of a vapor-gas mixture were carried out in a gas-discharge system, the scheme of which is shown in Figure 1. The design of such a system is described in detail in .
A special feature of this scheme is the use of an arc discharge with a self-heating hollow cathode and a sectional anode to ensure independent control of the metal evaporation rate and the current density of gas ions . One section was a water-cooled hollow cylinder made of stainless steel (12X18H10T), the second section was not water-cooled and consisted of a hollow crucible made of refractory material, closed on the end and sides with screens made of molybdenum and ceramic tube for better thermal insulation.
At the initial stage, before switching to the thermionic mode, the SHHC was heated in a glow discharge in a pulsed-periodic gorenje mode. Then the discharge switched to the arc mode, the Ar flow was 30 cm3/min. To independently adjust the flow of Al vapors, the ion current density, the degree of activation of the vapor-gas mixture, and the current to the cooled anode (Id) varied in the range of 5–30 A, the current in the crucible circuit (Icr) in the range of 1–10 A, the flows of reactive gases N2/O2 5–45 cm3/min, and the flows of HMDS/TEOS 0–2 g/h.

Figure 1 - Electrode scheme of the experimental facility
The composition of low-pressure arc discharge plasma (0,5–1 mTorr) with SHHC in gas mixtures of Ar+N2+Al and Ar+O2+Al with vapors of organosilicon precursors hexamethyldisilazane (HMDS) and tetraethoxysilane (TEOS), respectively, was analyzed by optical emission spectroscopy using an OceanOptics HR2000 spectrometer in the wavelength range from 200 up to 1100 nm (resolution 0,84 nm).
3. Results and discussion
The processes of activation of all components of the vapor-gas mixture, including the dissociation of reactive gas molecules and the decomposition of organosilicon precursors, are important factors influencing the formation of coatings based on PDC ceramics by plasma chemical deposition in a reactive vapor-gas medium. The plasma was analyzed by optical emission spectroscopy to identify its constituent components, as well as to assess the degree of dissociation of reactive gases N2 and O2 and the intensity of the decomposition of organosilicon precursors HMDS and TEOS.
Optical emission spectra of gas discharge plasma in mixtures of Ar+Al+N2+HMDS and Ar+Al+O2+TEOS are shown in Figure 3. In the UV region and in the visible range, there are mainly intense lines of excited Ar atoms (700-1000 nm), an Al line of 396.2 nm corresponding to the 3s23p–3s24s transition, as well as lines C (484,2 nm, 2s22p3p–2s22p(2P°1/2)16d) and H (656,3 nm, 2s–3p). The presence of the latter two indicates a sufficiently deep decomposition of the initial HMDS and TEOS molecules. The difference between the spectra in Figures 3 (1) and 3 (2) is the presence of atomic components resulting from the dissociation of reactive gases N2 and O2, respectively. Thus, N atoms (746.8 nm, 2s22p3(2D°)3s–2s22p3(2D°)s3p) were detected in Ar+N2+Al+HMD, in Ar+O2+Al+TEOS - O (794,8 nm, 2s22p3(2D°)3s–2s22p3(2D°)3p). In addition to the neutral component of the plasma, lines of ionic components were detected on the spectra: Ar+ (400–450 nm), and in the vapor-gas mixture Ar+O2+Al+The lines Al+ (390,1 nm, 3s23p–3p2) and N2+ (391,9 nm, 2s22p3(2D°)3s–2s22p3(4S°)3p) were also present in the TEOS. Of the molecular components, the singuletes N2 (337,1 nm, С3Πu–B3Πg) and O2 (759,37 nm, b1Σg+–X3Σg-) were detected in the spectra.
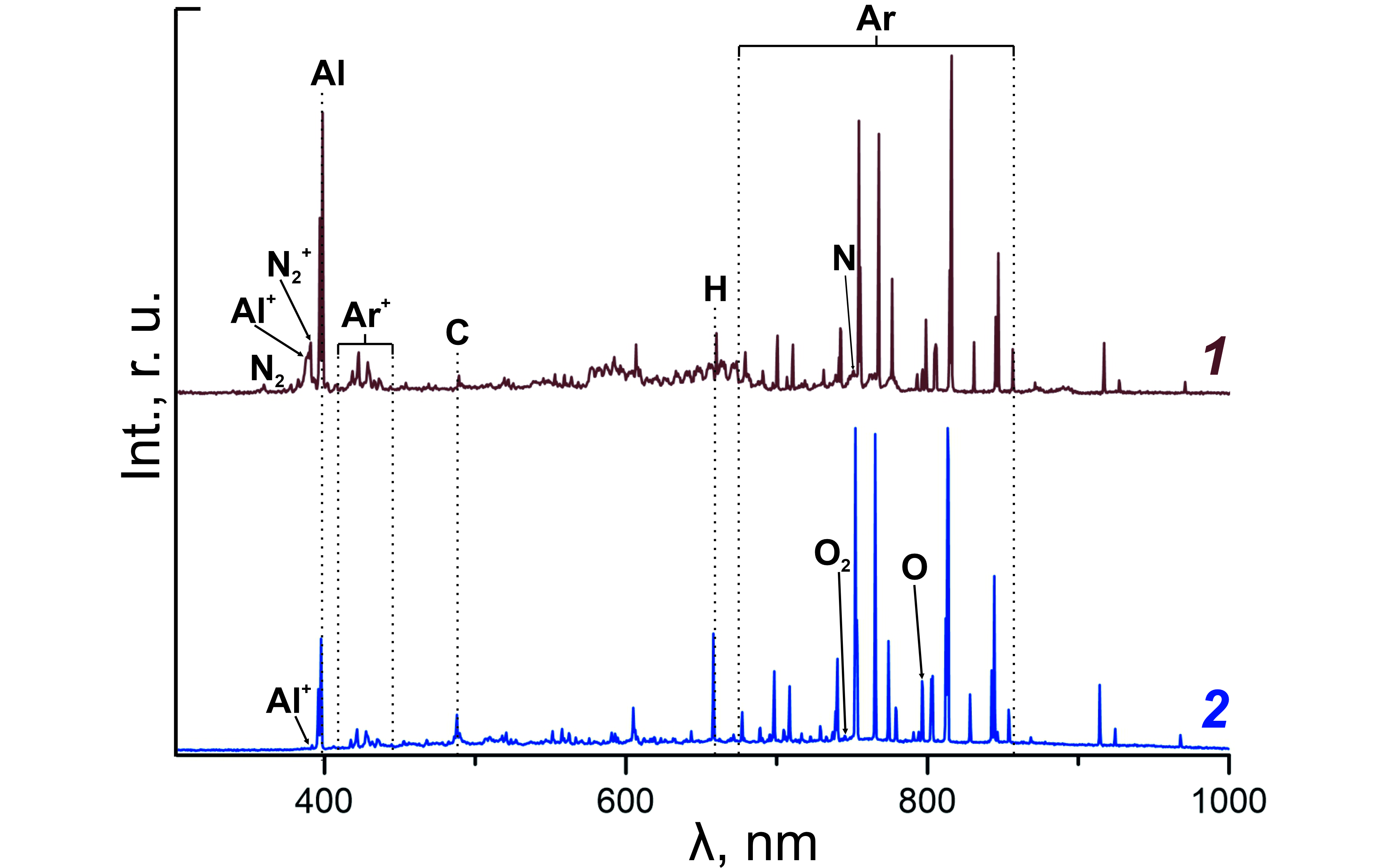
Figure 2 - Optical radiation spectrum of Ar+N2+Al+HMDS (1) and Ar+O2+Al+TEOS (2) arc plasma
The dependences of the degree of dissociation of reactive gases N2 and O2 on various conditions of discharge combustion are shown in Figure 3. As can be seen, with this method of activation of a vapor-gas medium, the degree of nitrogen dissociation in a mixture of Ar+N2+Al+HMDS is quite high compared, for example, with RF methods used to generate nitrogen atoms . Since such systems are dominated by ionic components formed in corona and glow discharges, which require a high concentration of ions to maintain.
Table 1 - Data used in calculation
Particle grade | λ, nm | A×107, s–1 | hν, eV | τ×10–9, s | σ, cm2 | A×τ |
N | 746,8 | 1,96 | 11,99 | 26,3 | 6,2×10–16 | - |
N2 | 337,1 | - | 11,1 | - | 5,5×10–18 | 0,52 |
O | 794,8 | 0,58 | 12,5 | 4 | 3×10–18 | - |
O2 | 749,4 | - | 1,63 | - | 4,6×10–18 | 0,33 |
H | 656,3 | 4,41 | 12,09 | 1,6 | 9,3×10–17 | - |
Ar | 811,5 | 3,3 | 13,07 | 3,1 | 5×10–18 | - |
An increase in the discharge current in the range of 5–25 A leads to a slight (5–10%) increase in the degree of dissociation. To a much greater extent, the degree of nitrogen dissociation in the sample location is influenced by the current in the crucible circuit, which can be explained by a combination of a high concentration of electron flux near the crucible and a high pressure of metal vapors in the area of their drift from the crucible towards the substrate. As is known , nitrogen dissociation in plasma in the presence of a stream of fast electrons occurring in a discharge with SHHC occurs mainly as a result of a two-step process of dissociative recombination of molecular nitrogen ions: e-+N2→N2++2e–, e–+N2+→2N. The presence of a dense vapor stream of a more easily ionized metal probably intensifies the process of formation of molecular ions by recombination with the participation of metal ions.
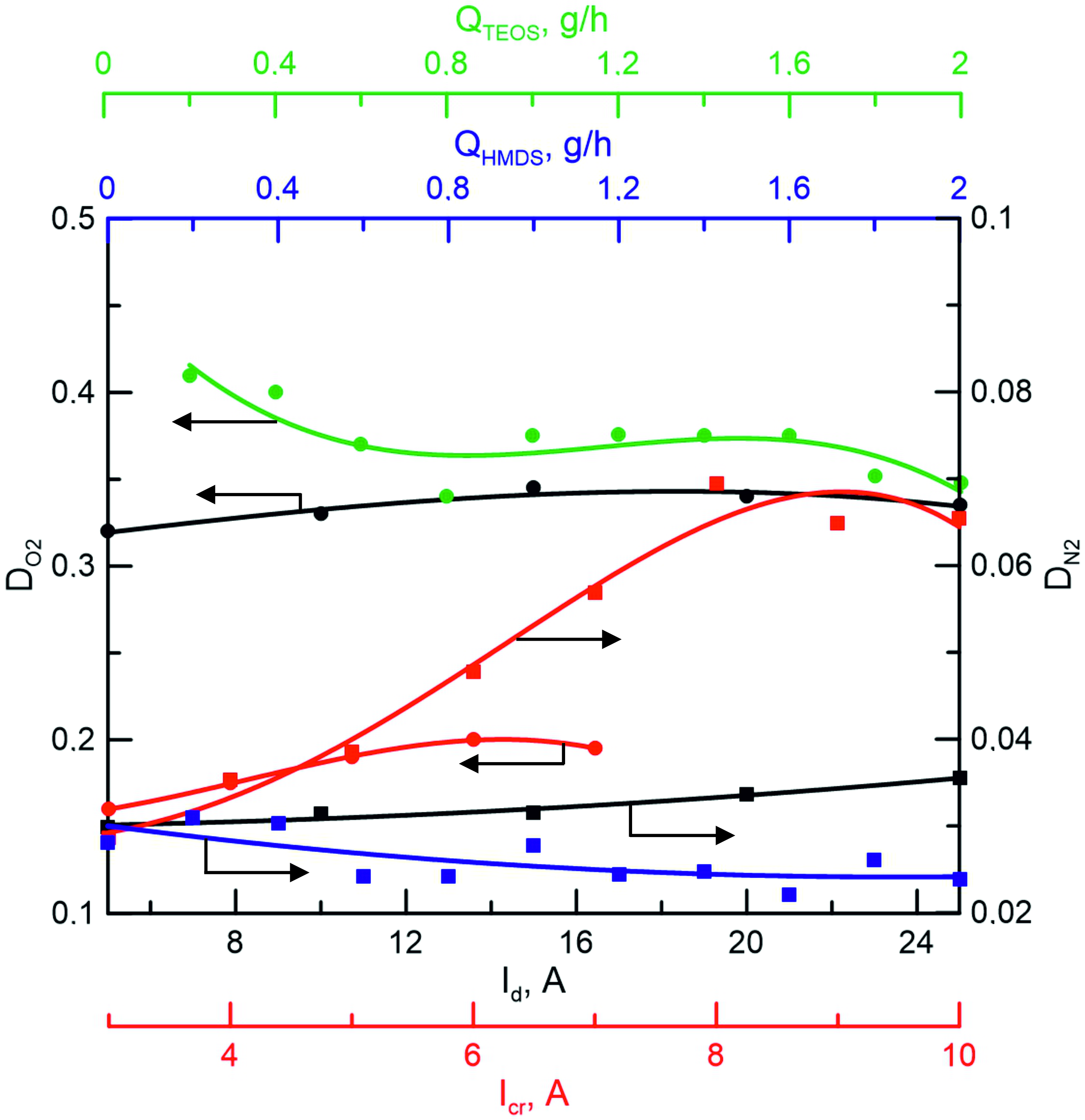
Figure 3 - Dependences of the degree of dissociation of nitrogen DN2 and oxygen DO2 on the discharge current (Id), crucible current (Icr), and precursor flux
Figures 4 and 5 show the dependences of the intensities of the hydrogen line H (656,3 nm) on various plasma conditions. The course of HMDS decomposition reactions occurring in a gas-discharge plasma is determined by the binding energies between the atoms in the initial molecules, as well as the discharge power. The decomposition of the hexamethyldisilazane molecule in plasma occurs as a result of a stepwise reaction: first, the Si-C bond breaks and the methyl group is lost, then the Si-N single bond breaks. As a result, fragments of a molecule with a Si=N double bond appear in the plasma, which are involved in the formation of coatings. In the following stages, the stronger N-H and C-H bonds and the Si=N double bond are destroyed, resulting in the appearance of H, C, and Si atoms in the plasma, the content of which can be used to determine the degree of decomposition of HMDS.
It can be seen that the current in the crucible circuit most significantly affects the decomposition of the initial precursor molecules in the Ar+N2+Al+HMDS medium, which can be explained by an increase in pressure in the local region near the crucible, where intensive activation processes of all plasma-forming components occur due to an increase in the frequency of particle collisions in the plasma. The influence of other parameters turned out to be less significant. A slight decrease in the decomposition rate of the HMDS molecule with an increase in the N2 flux may be due to an increase in the overall pressure in the vacuum chamber and a corresponding decrease in the average electron energy.
Just as in the case of HMDS, the decomposition of the TEOS molecule occurs stepwise: first, the Si-O bond breaks to form C2H2-O, followed by the loss of the C2H5 methyl group, but to date little is known about the intermediate products of the decomposition reaction of the TEOS molecule. It is reported that the decomposition of TEOS and the formation of SiCO-based films are affected by the addition of O2, which accelerates the decomposition of organosilicon molecules
. Atomic oxygen has a significant effect on the quality of the coatings obtained, since there is a relationship between the substrate temperature and the deposition rate, which strongly depends on the presence of O: the deposition rate increases with increasing substrate temperature in the presence of oxygen. However, the formation of coatings of the desired chemical composition with a certain set of properties depends not only on the introduction of a reactive gas, but also on the decomposition process of the TEOS molecule, which also contains oxygen. As can be seen in Figure 5, an increase in the magnitude of the TEOS flow leads first to an increase in the intensity of H, and then to its decrease. The gas-phase decomposition reaction of the TEOS molecule can also be enhanced by atomic oxygen, as in , which can be observed in our case.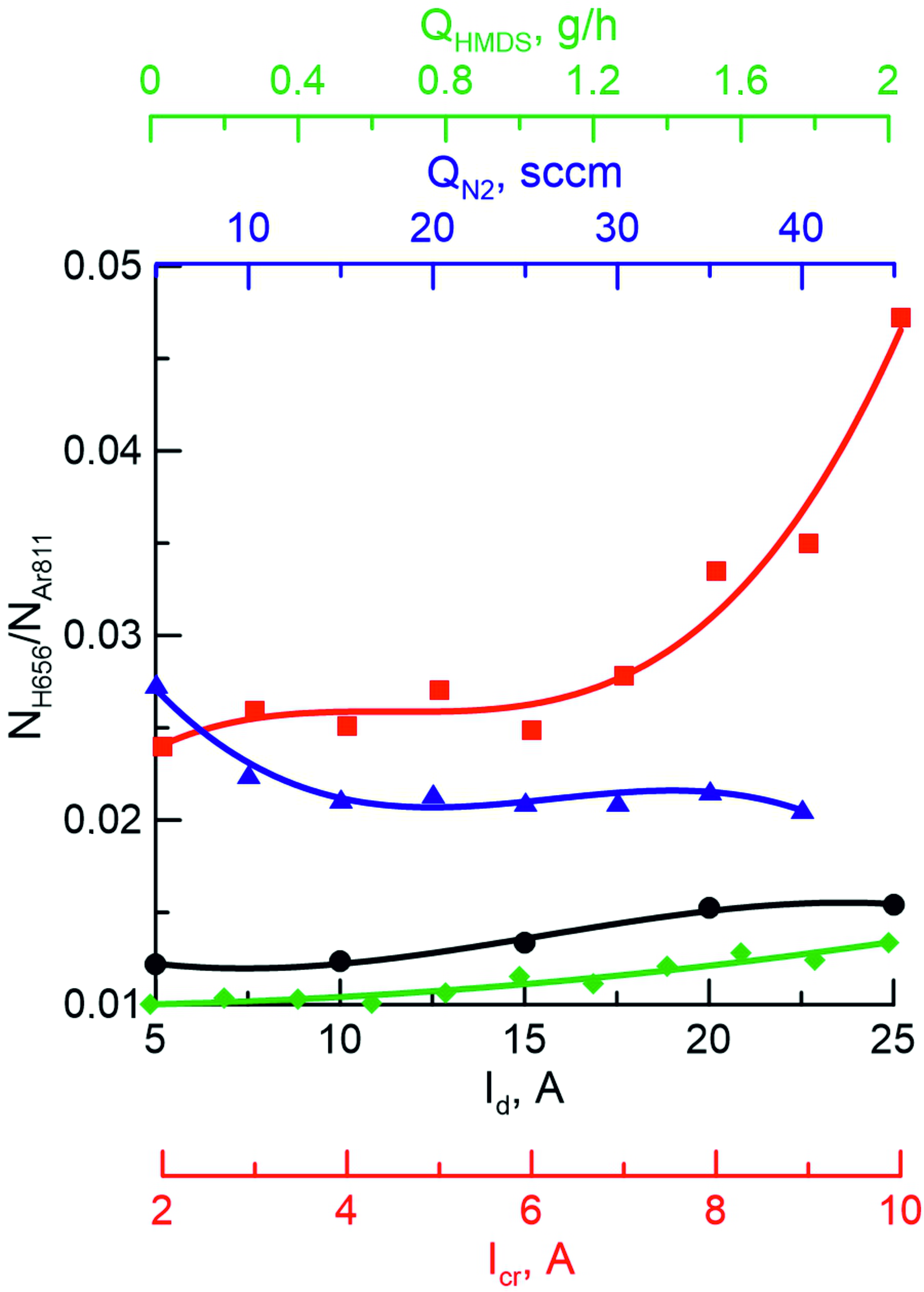
Figure 4 - Dependence of the ratio of hydrogen to argon (NH656/NAr811) on the discharge current (Id), crucible current (Icr), nitrogen flux (QN2) and hexamethyldisilazane flux (QHMDS) in a mixture of Ar+N2+Al+HMDS
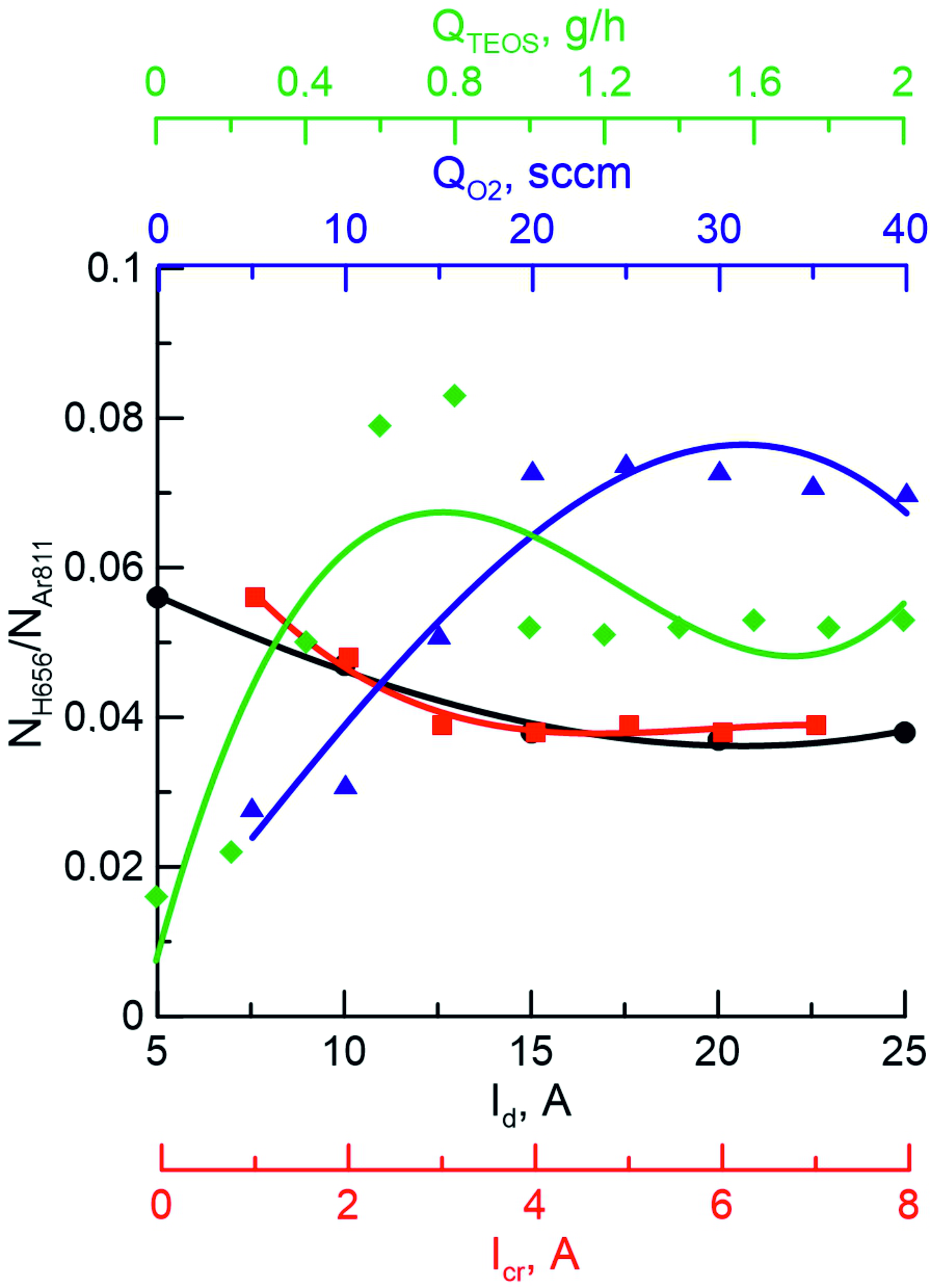
Figure 5 - The dependence of the ratio of hydrogen to argon (NH656/NAr811) on the discharge current (Id), crucible current (Icr), oxygen flux (QO2) and tetraethoxysilane flux (QTEOS) in a mixture of Ar+O2+Al+TEOC
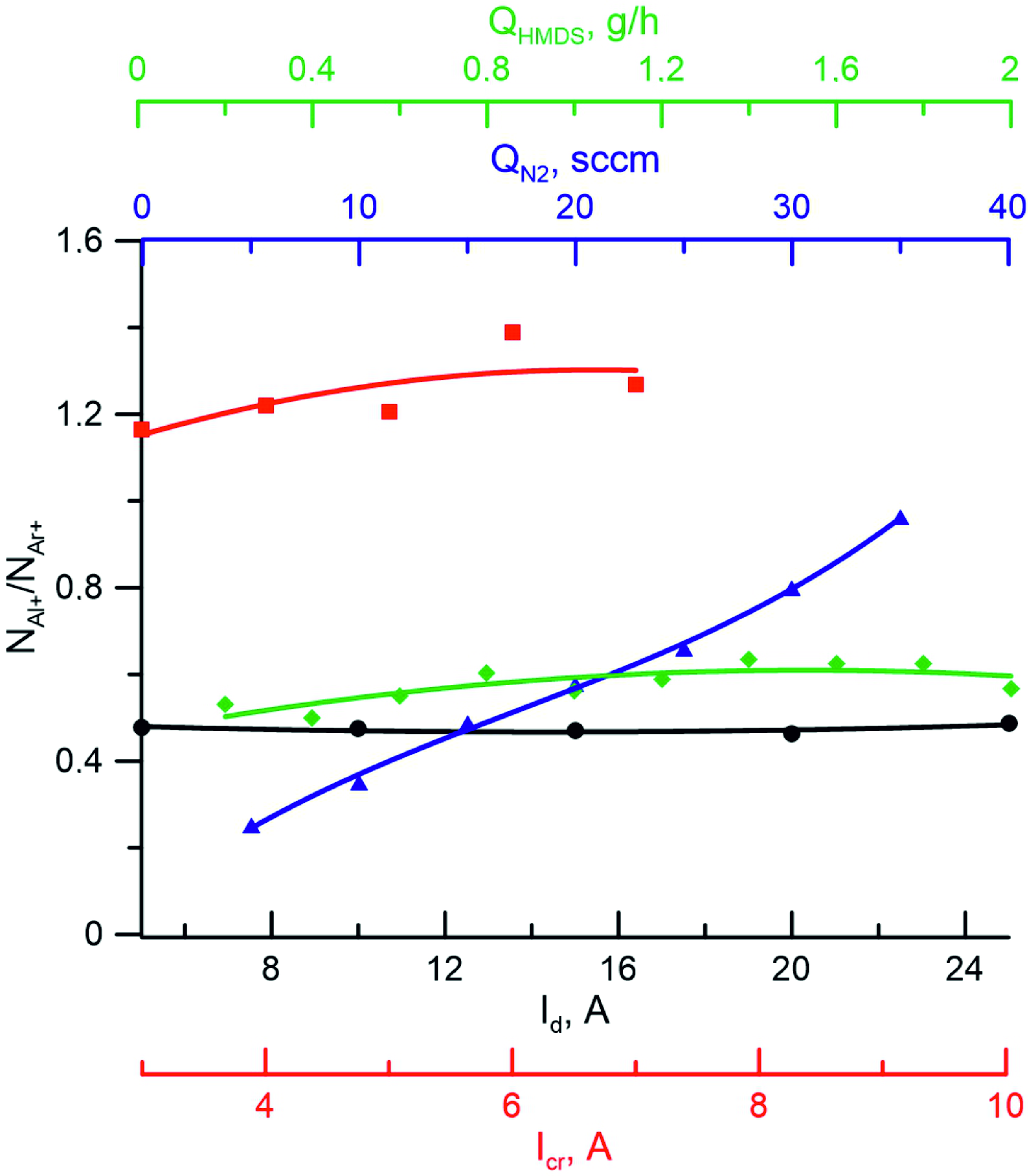
Figure 6 - Dependences of the ratio of concentrations of aluminum ions and argon ions (NAl+/NAr+) on the discharge current (Id), crucible current (Icr), nitrogen flux (QN2) and hexamethyldisilazane flux (QHMDS) Ar+N2+Al+HMDS
Figure 7 shows the dependences of the ratio of concentrations of aluminum ions (NAl+) and argon ions (NAr+) on various discharge parameters obtained in the medium Ar+O2+Al+TEOC. The current in the crucible circuit has the most significant effect on this ratio. As can be seen, the dependence has a nonmonotonic character: the decrease in NAl+/NAr+ from 0,8 to 0,6 with an increase in current from 1 to 4 A is due to the constant intensity of the Al+ line, which indicates a low concentration of aluminum atoms evaporating from the surface of heated aluminum. At the same time, there is an increase in the intensity of the Ar+ line as the discharge power increases. A further increase in NAl+/NAr+ is associated with an increase in the temperature of the crucible and a significant increase in the concentration of Al vapors and their subsequent ionization. This nonmonotonic behavior is explained by the nonlinear dependence of the saturated vapor pressure on the metal temperature, therefore, in the 1–4 A region at lower temperatures and, consequently, saturated vapor pressures, the concentration of aluminum atoms remains low. Probe diagnostics of the plasma showed that the ion current density in the sample location region at maximum currents in the studied ranges can reach 10–12 mA/cm2.
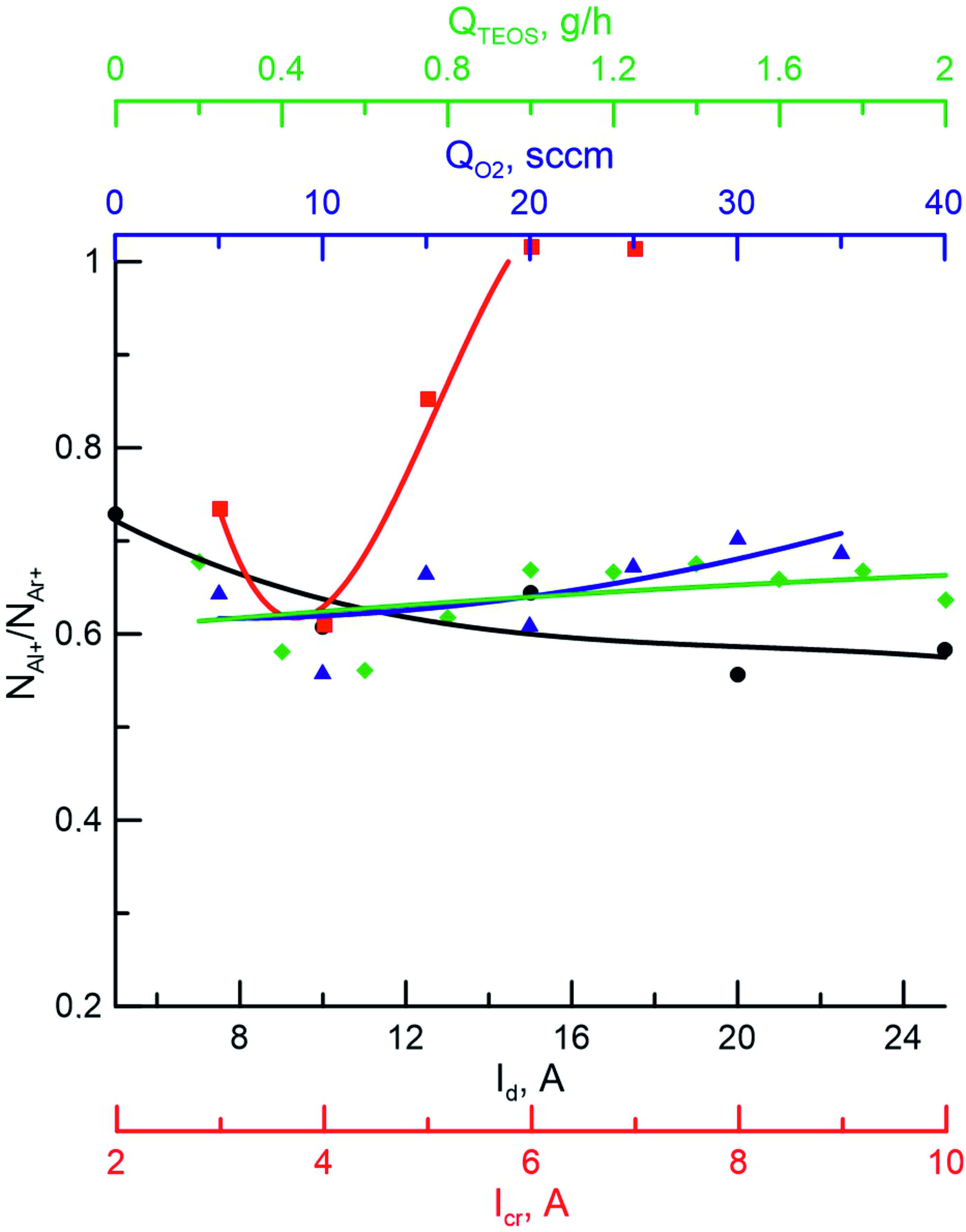
Figure 7 - Dependences of the ratio of concentrations of aluminum ions and argon ions (NAl+/NAr+) on the discharge current (Id), crucible current (Icr), nitrogen flux (QO2) and hexamethyldisilazane flux (QTEOS) Ar+O2+Al+TEOS
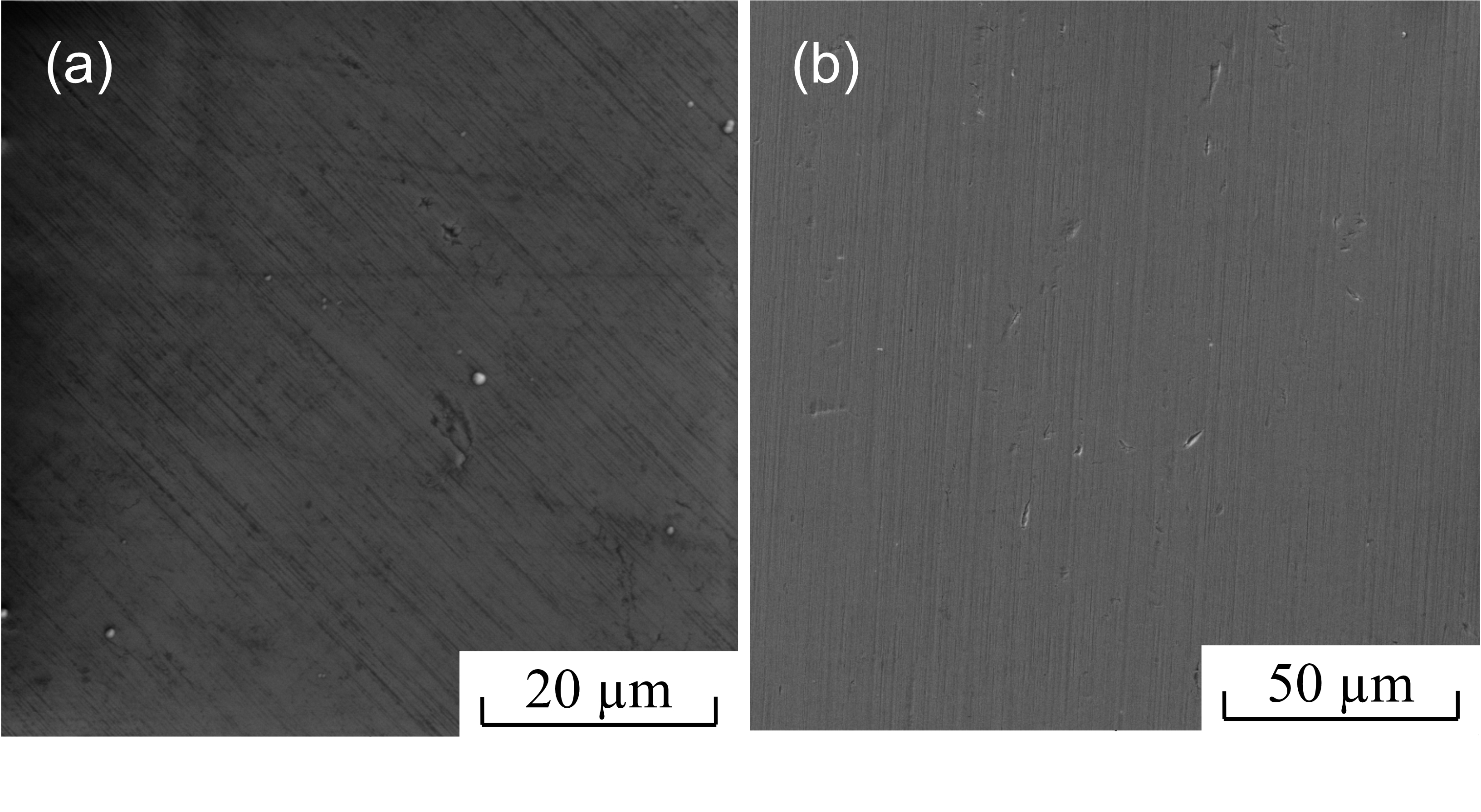
Figure 8 - SEM images of the surface of SiAlCN (a) and SiAlCO (b) films
4. Conclusion
Thus, the method of activation of a vapor-gas mixture containing organosilicon precursors and additional reactive components N2 and O2 in an arc discharge with a hollow self-heating cathode and a sectional anode under aluminum evaporation conditions, as well as the effect of combustion modes on the dissociation of reactive gases N2 and O2 and the degree of decomposition of HMDS and TEOS, was investigated. The results of spectral plasma diagnostics showed the presence in the studied media of the necessary elements for the synthesis of SiAlCN and SiAlCN coatings. High rates of decomposition of the initial HMDS and TEOS molecules, high values of the degree of dissociation of N2 up to 7% and O2 up to 40% were achieved. The investigated method of activation of a vapor-gas medium provides an intense ion flow to the treated surface with a fraction of metal ions in excess of 50%, which is important for the formation of dense, high-quality coatings. Trial SiAlCN and SiAlCO coatings were obtained in combined-cycle mixtures of Ar+N2+Al+HMDS and Ar+O2+Al+TEOS, respectively. Thus, the results of the study showed the prospects of using this synthesis method to obtain PDC coatings with the necessary elemental composition and the required set of physico-chemical properties.
