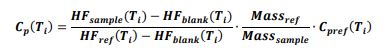PREPARATION OF AMORPHOUS OXIDES AL2O3 AND FE2O3 BY A MODIFIED SOL-GEL METHOD
DOI:https://doi.org/10.23670/IRJ.2022.119.5.070
PREPARATION OF AMORPHOUS OXIDES AL2O3 AND FE2O3 BY A MODIFIED SOL-GEL METHOD
Research article
Derevyanko M.S.1, *, Cheverikin V.V.2, Bajenova I.A.3, Kuzovchikov S.V.4, Kondratiev A.V.5
1, 2, 3, 4, 5Thermochemistry of Materials Scientific Research Centre, Moscow, Russia
*Corresponding author (maksim.derevyanko.96[at]mail.ru)
Abstract
A new approach to obtain amorphous oxides was investigated both theoretically and experimentally using X-ray diffraction (XRD), electron probe microanalysis (EPMA) techniques. In this work, the conventional sol-gel method was modified by the addition of malic acid as an oxidizing agent and successfully applied to obtain two oxides Al2O3& Fe2O3 with the amorphous structure. Thermal stability and the enthalpy of fusion of the obtained amorphous oxides were examined by the differential scanning calorimetry (DSC). It was found that amorphous Al2O3 was stable up to 790 – 810 °C, while amorphous Fe2O3 recrystallized at about 160 – 180 °C, depending on the heating rate. The heat capacity using the DSC method has been measured. The results were observed depending on the heating temperature.
Keywords: Sol-gel process, Al2O3, Fe2O3, Amorphous structure.
ПОЛУЧЕНИЕ АМОРФНЫХ ОКСИДОВ AL2O3 И FE2O3 МОДИФИЦИРОВАННЫМ ЗОЛЬ-ГЕЛЬ МЕТОДОМ
Научная статья
ДеревянкоМ.С.1, *, Чеверикин В.В.2, БаженоваИ.А.3, КузовчиковС.В.4, КондратьевА.В.5
1, 2, 3, 4, 5 Национальный исследовательский технологический университет, Москва, Россия
* Корреспондирующий автор (maksim.derevyanko.96[at]mail.ru)
Аннотация
Новый подход к получению аморфных оксидов исследован как теоретически, так и экспериментально с использованием методов рентгеновской дифракции (РФА), электронно-зондового микроанализа (МРСА). В этой работе традиционный золь-гель метод был модифицирован добавлением яблочной кислоты в качестве окислителя и успешно применен для получения двух оксидов Al2O3 и Fe2O3 с аморфной структурой. Термическую стабильность и энтальпию плавления полученных аморфных оксидов исследовали методом дифференциальной сканирующей калориметрии (ДСК). Установлено, что аморфный Al2O3 стабилен до 790‒810 °С, а аморфный Fe2O3 рекристаллизуется примерно при 160‒180 °С в зависимости от скорости нагрева. Измерена теплоемкость методом ДСК. Результаты наблюдались в зависимости от температуры нагрева.
Ключевые слова: Золь-гель процесс, Al2O3, Fe2O3, Аморфная структура.
- Introduction
Sol-gel method is used in many fields and areas of modern chemistry to produce materials from solutions, an essential element of which is the formation of a gel at one of the stages of the process 1. Often, in order to obtain compounds by this method, it is necessary to use relatively expensive reagents (e.g. metal alkoxides 2).Fortunately, there are universal methods of obtaining various compounds using cheaper and more readily available reagents that produce the expected results, but in an easier and less costly way.
Aluminum oxide is known to be amphoteric 3and has many allotropic modifications 4. High-temperature α-Al2O3 or corundum 5is the only thermodynamically stable form of Al2O3 occurring in nature 6. The compound, sometimes referred to as β-Al2O3, in fact is not pure aluminum oxide, but a solution that contains several aluminates of alkali and alkaline earth metals 7.
In addition, there are low-temperature modifications of aluminum oxide 8. The widely known γ-modification of Al2O37, also called active aluminum oxide, has a lower melting point. And the amorphous form, the production of which is more attractive for us for further use in our experiments.
Iron oxide (III), just like aluminum oxide, is an amphoteric oxide 9, which in turn has many basic properties. In terms of the range of its thermal properties, Fe2O3 attracts specifically by the fact that it has a low melting point of the order of 1600 °C 10, that can be useful in terms of investigating its various relaxation properties.
In the present paper a modified sol-gel method was used to obtain amorphous forms of Al2O3 and Fe2O311, which was confirmed by XRD and EBSD techniques. Purity of the obtained samples was examined by EPMA method. The study of the thermodynamic properties of the obtained compounds was the main focus of this work.
Thermal stability of amorphous oxides was determined using the DSC method. A conventional method of thermal decomposition of the corresponding nitrates was also used for comparison.
- Experimental
- Overview
The compounds described in the previous section, namely the amorphous forms of aluminum and iron oxides, can and have been obtained from the salts corresponding to these elements - nitrates 12, 13. However, in this study, non-standard approach and aspects of the sol-gel method were used to obtain the corresponding oxides 1.
At first, attempts to obtain oxides by an improved sol-gel method and their further analysis were made. Then, the conventional thermal decomposition of aluminum 12and iron 13 nitrates in an electric furnace was used to compare the products of these two reactions and clearly show their differences.
- Modified sol-gel method
Sol-gel technique represents a large group of methods for synthesizing materials from solutions, an essential element of which is the formation of a sol at one stage and a gel at another stage of the process. In the present work, an improvement of the traditional sol-gel method was proposed, which consisted in the addition of malic acid as an oxidizer to obtain oxides 11with an amorphous structure.
Malic acid is a hydroxycarboxylic acid, which has good oxidizing properties, promoting thereby separation and removal of nitrogen dioxide from the reaction with the nitrate salts used as reagents in this method. Also, the use of malic acid lies in its manifestation of the properties of a natural preservative, which can prevent the appearance of harmful substances in the reaction products, as well as slow down their chemical oxidation with atmospheric oxygen.
The corresponding oxides were obtained by the improved sol-gel method and also by traditional thermal decomposition of aluminum and iron nitrates for comparison of these two methods. The approach proved to obtain an amorphous structure of oxides, in addition, having particle sizes at the nanoscale.
Further modifications of this approach are associated with low operating temperatures of the synthesis and unpretentious conditions for its implementation.
- Sample preparation
- Al2O3
Modified sol-gel method:
Raw material with purity Al(NO3)3⋅9H2O (98 wt.%) was dissolved in distilled water in a ratio of 1:5 (10 g of nitrate to 50 ml of water) with vigorous stirring and room temperature. After complete dissolution, malic acid (food additive E296) was added in an amount of 3 g. The resulting solution was put in a thermostat preheated to 80 °C for 3 hours. Then, solution of a translucent turquoise color was thermostated at 60 °C for 24 hours. The product was a yellowish foamy mass, covered with a crust. The resulting compound was dried again at 80 °C, but for 19 hours to obtain crystals of a foamy state of turquoise color.
The crystals were placed in a beryllium crucible and heated up in a muffle furnace to 700 °C with the heating rate about 20-25 K/min, then the sample was sintered at 700 °C for 15 minutes.
The small fraction was sampled, let the rest of the sample heat up to 850 °C and keep it at this temperature for another 30 min. The muffle furnace was turned off and cooled to room temperature with the sample inside. Then the sample was removed, and the resulting grayish powder was examined using the EPMA and XRDmethods.
Thermal decomposition of the corresponding nitrate:
A small amount of aluminum nitrate was poured into a porcelain glass. The glass with the sample was placed on a preheated electric oven located under the vent hood. The onset of the reaction can be seen by abundant emission of a brown gas (NO2). The melting process was continued until the compound was completely melted and nitrogen dioxide ceases to evolve.
After that, the glass was removed from the oven and cooled in air under the hood. The abundant crystallization of the resulting compound was noticed. The fine-crystalline white powder with a grayish tint was obtained. The structure and composition of amorphous Al2O3 were later confirmed by XRD and EPMA.
- Fe2O3
Modified sol-gel method:
After the successful completion of the synthesis with aluminum oxide, to test the method on another amphoteric oxide was decided. Fe2O3 was the most promising for this 9.
Based on the ratio of equivalents of aluminum nitrate and malic acid, the weight of the sample for iron nitrate (III) was calculated. Raw material with purity Fe(NO3)3⋅9H2O (98 wt.%) was dissolved in distilled water in a ratio of 1:5 (11 g of nitrate to 55 ml of water) with vigorous stirring and room temperature. After complete dissolution, malic acid (food additive E296) was added in an amount of 3.5 g. The resulting brown solution was put in a thermostat preheated to 80 °C for 3 hours. Then, solution was kept at 60 °C for 24 hours. A wet, powdery brown mass was obtained. After 24 hours, the resulting compound was dried again at 80 °C, but for 20 hours. The brown crystals were got.
The brown crystals were sintered in a muffle furnace, having previously given a sample for XRD and EPMA. The sample was placed into a beryllium crucible and heated up in the furnace to 150 °C, according to the data obtained from 14. By sintering compound at a given temperature for 20 minutes, a fraction for XRD and EPMAanalyses was separated to determine the chemical and phase compositions of the sample. The powdered crystals of a dark burgundy color were obtained.
In the same way the fractions of the sample thermostated at temperatures of 600 °C (30 minutes) and 850 °C (30 minutes) were proceeded.
Thermal decomposition of the corresponding nitrate:
A small amount of Fe(NO3)3 was poured into a porcelain glass. The glass with the sample was placed on a preheated electric oven located under the vent hood. The onset of the reaction can be seen by abundant emission of a brown gas (NO2). The melting process was continued until the compound was completely melted and nitrogen dioxide ceases to evolve.
After that, the glass was removed from the oven and cooled in air under the hood. The abundant crystallization of the resulting compound was noticed. A fine-crystalline brown powder was obtained. The structure and composition of amorphous Fe2O3 were later confirmed by XRD and EPMA.
- Microstructure analysis
An electron probe microanalysis (EPMA) was carried out using a TESCAN VEGA LMH microscope with a LaB6 cathode and an energy dispersive (EDS) X-ray microanalysis system (Oxford Instruments Advanced Aztec Energy) and a four-crystal wave spectrometer, with the acceleration voltage of 20 kV. The detector was calibrated using the standard samples of Al (99.99%) and Fe (99.999%) from Oxford Instruments. The chemical composition was determined by comparing the characteristic radiation observed and incorporated into the analyzer. Quantitative analysis was carried out on the basis of differences in the radiation intensity of the strongest line of the K- or L- series of characteristic radiation of the element. The measurement error in determining the concentration of elements using EPMA was 0.1 at.% 15.
- XRD analysis of samples
X-ray diffraction (XRD) analysis was applied to determine the phase compositions of the alloys using Cu Kα radiation operated at a voltage of 40 kV, a current of 40 mA and filtered with a Ni-crystal monochromator. XRD measurements were performed using a multipurpose X-ray diffractometer (Bruker-AXS D8 Discover). Al2O3 was used as an internal standard to achieve better accuracy in the 2θ values. A parallel beam with a divergence of 0.03° was formed using the mirror of Gobel. The reflected intensity of the beam was measured using a LYNXEYE position sensitive detector (angular resolution of 0.015°). The phases were identified by comparing their diffraction patterns with those known from the literature or calculated using the WinXPOW and PowderCell software packages 16. The lattice parameters were calculated with the PowderCell software based on the least-squares method.
- Calorimetric measurements
- Differential thermal calorimetry
Differential thermal calorimetry (DTC) was carried out using a DSC LabSys Evo (Setaram / KEP Technologies). The pure metal standards: Al (99.995%), Au (99.99%) and Ag (99.998%) were used for calibration. Samples were placed in an Al2O3 crucible and experiments were carried out under 20 ml/min flow of high-purity argon (99.998%). The heating and cooling rates were 1, 5, 10, 15 and 20 K/min, respectively.
- Heat capacity
The heat capacity data of amorphous Al2O3 sample was measured with a DSC LabSys Evo Setaram using the detector 3D Cp Sensor. The output of the calorimeter readings and the calculation of the heat capacity were carried out using the Calisto software. The sample was weighed on an analytical balance to the nearest decimal fraction of a gram. The heat capacity measurements were carried out in the temperature range 473 - 1023 K. The experiment was carried out in a platinum crucible in an argon flow (99.999%) of about 20 ml/min. The measurements consisted of three successive experiments: with an empty crucible (blank), with a standard reference sample with a known heat capacity in the investigated temperature range (reference), and with the sample under study. Compressed aluminum oxide (Setaram, France) was used as a standard sample. The following equation was used to calculate the heat capacity:
where HF is the signal received on the calorimeter, mV;
Mass– mass, g;
Сp– heat capacity, J·mol-1·K-1;
blank –for an empty crucible;
ref – for a standard sample;
sample– for the test sample.
- Results and discussion
- Structure analysis
All synthesized samples were obtained in two ways at different temperature ranges. A suitable method for the preparation of amorphous compounds, properties of oxides and their application are discussed in this section.
- Al2O3
Modified sol-gel method:
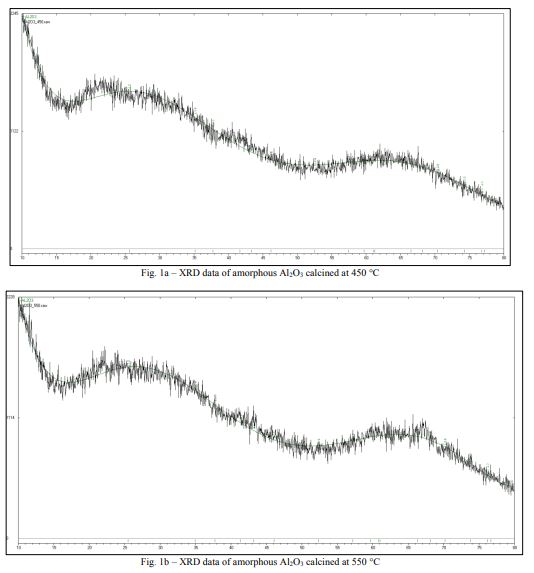
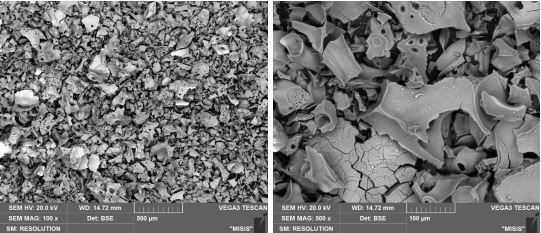
The data of the composition and morphological structure of the oxides were confirmed using the method of X-ray diffraction analysis (XRD) and electron microscopy. From the XRD results the powders calcined at 450, 550, and 650 °C were X-ray amorphous Al2O318(Fig. 1a, b, c), which was also evidenced by the EBSD analysis data. Details of the process are described in the EBSD section.
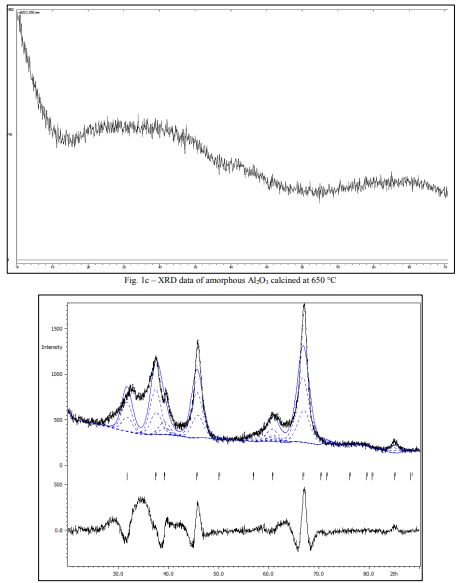
The powder, heat-treated at 850 °C was represented mostly γ-Al2O37(Fig. 1d), as evidenced by XRD data.
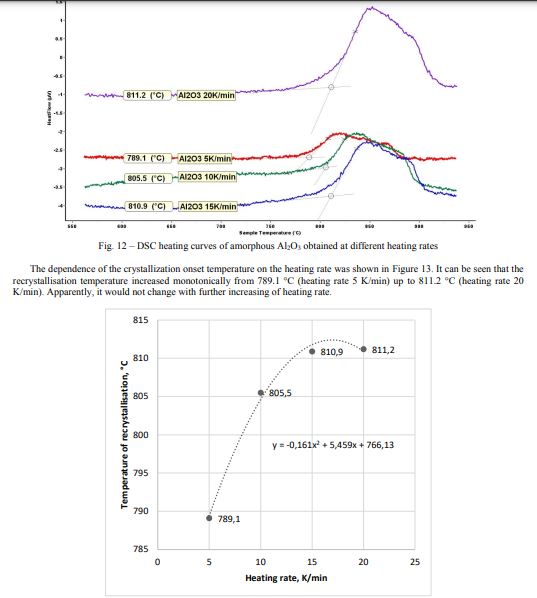
The amorphous structure of Al2O3 can be obtained at temperatures 450-650 oC, as evidenced by Figures 1a-c. It should be noted that the process of recrystallization of amorphous Al2O3 started at T=850 oC (Figure 1d).
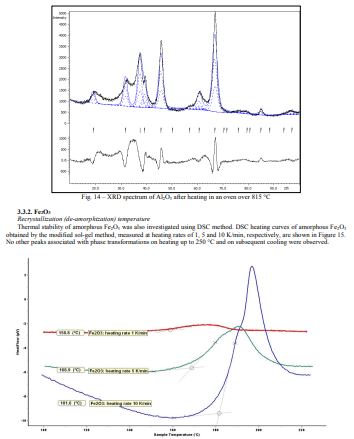
The spectrum from Fig. 1d can partly be identified as a phase with the structure type H1.1. (cF56/2) or -Al2O3. The broadness and intensities of the peaks do not fully coincide with the CIF peaks due to the presence of various types of defects in the structure 19and possible presence of an amorphous or/and other crystalline forms of Al2O3.
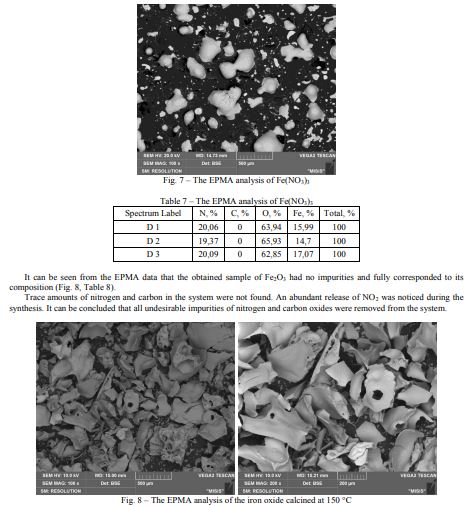
Purity of the Al2O3 samples obtained by the sol-gel method and sintering at different temperatures was analyzed using the EPMA method. First of all, the aluminum nitrate was examined for the presence of impurities. The EPMA data showed that Al(NO3)3 had a small Na impurity, which can be expected based on the grade of the compound purity (Fig. 2, Table 1).
Table 1 – The EPMA analysis of Al(NO3)3
| Spectrum Label | N, % | C, % | O, % | Na, % | Al, % | Total, % |
| S 1 | 11,97 | 0 | 67,52 | 20,5 | 100 | |
| S 2 | 11,83 | 0 | 68,54 | 19,63 | 100 | |
| S 3 | 11,38 | 0 | 69,04 | 19,58 | 100 | |
| S 4 | 18,09 | 0 | 58,28 | 3,1 | 20,52 | 100 |
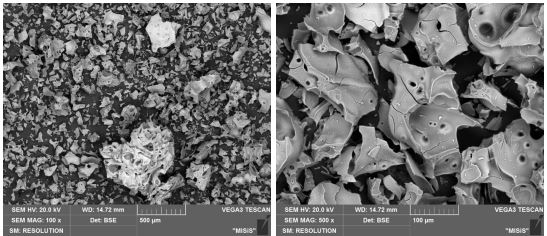
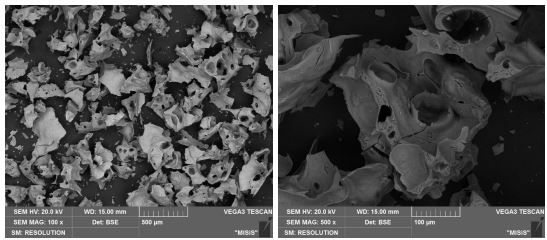
The morphology and purity of the obtained oxides were shown in Figures 3a-d and Tables 2-5. A scaly morphology with large pores and a size of 200-300 µm was observed in amorphous Al2O3 particles., while the crystalline Al2O3 particles were bigger, about 0.5-1 mm.
Trace amounts of the admixture of nitrogen and carbon in the system were not found. An abundant release of NO2 was noticed during the synthesis. It can be concluded that all undesirable impurities of nitrogen and carbon oxides were removed from the system.
Table 2 – The EPMA analysis of the amorphous Al2O3 (450 °C)
| Spectrum Label | N, % | C, % | O, % | Al, % | Total, % |
| S 1 | 0 | 0 | 59,06 | 40,94 | 100 |
| S 2 | 0 | 0 | 64,77 | 35,23 | 100 |
| S 3 | 0 | 0 | 69,83 | 30,17 | 100 |
| S 4 | 0 | 0 | 61,4 | 38,6 | 100 |
Fig. 3b – The EPMA analysis of the amorphous Al2O3 (550 °C)
Table 3 – The EPMA analysis of the amorphous Al2O3 (550 °C)
| Spectrum Label | N, % | C, % | O, % | Al, % | Total, % |
| S 1 | 0 | 0 | 54,8 | 45,2 | 100 |
| S 2 | 0 | 0 | 66,34 | 33,66 | 100 |
| S 3 | 0 | 0 | 64,22 | 35,78 | 100 |
| S 4 | 0 | 0 | 64,61 | 35,39 | 100 |
| S 5 | 0 | 0 | 64,81 | 35,19 | 100 |
Fig. 3c – The EPMA analysis of the amorphous Al2O3 (650 °C)
Table 4 – The EPMA analysis of the amorphous Al2O3 (650 °C)
| Spectrum Label | N, % | C, % | O, % | Al, % | Total, % |
| S 1 | 0 | 0 | 67,1 | 32,9 | 100 |
| S 2 | 0 | 0 | 66,04 | 33,96 | 100 |
| S 3 | 0 | 0 | 67,74 | 32,26 | 100 |
| S 4 | 0 | 0 | 44,53 | 55,47 | 100 |
Fig. 3d – The EPMA analysis of γ-Al2O3 (850 °C)
Table 5 – The EPMA analysis of γ-Al2O3 (850 °C)
| Spectrum Label | N, % | C, % | O, % | Al, % | Total, % |
| D 1 | 0 | 0 | 61,9 | 38,1 | 100 |
| D 2 | 0 | 0 | 66,86 | 33,14 | 100 |
It was also found that, depending on the sintering temperature of the sample, different modifications of aluminum oxide can be obtained 4.
Thermal decomposition of the corresponding nitrate:
Along with the sol-gel method, the type of structure and composition of aluminum oxide obtained by thermal decomposition of nitrate on an electric stove from the corresponding nitrate were studied.
In the sol-gel method, after annealing at 450 oC, an amorphous oxide structure can be obtained. For these reasons, the temperature of the electric stove was 450 – 490 oC with a time of 20 minutes.
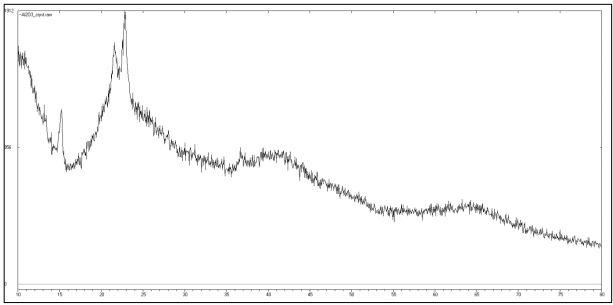
According to the XRD data, aluminum oxide obtained by thermal decomposition had a crystalline structure (Fig. 4). It can be concluded that an amorphous structure cannot be obtained by this method.
Fig. 4 – The XRD data of the aluminum oxide obtained by thermal decomposition
The purity and composition of the oxide has been proven based on EPMA data. A nitrogen impurity and the absence of trace amounts of carbon were found in the compound (Fig. 5, Table 6).
Fig. 5 – The EPMA analysis of the amorphous Al2O3 obtained by thermal decomposition
Table 6 – The EPMA analysis of the amorphous Al2O3 obtained by thermal decomposition
| Spectrum Label | N, % | C, % | O, % | Al, % | Total, % |
| S 1 | 10,2 | 0 | 75,74 | 14,06 | 100 |
| S 2 | 0 | 79,34 | 20,66 | 100 | |
| S 3 | 10,7 | 0 | 79,31 | 9,95 | 100 |
| S 4 | 11,8 | 0 | 70,56 | 17,66 | 100 |
| S 5 | 9,85 | 0 | 72,58 | 17,56 | 100 |
- Fe2O3
Modified sol-gel method:
Several samples were taken at different stages of obtaining iron oxide (III) at different temperatures. The fractions isolated during sintering at temperatures of 600 °C and 850 °C had a crystalline structure 20. Therefore, these compounds will not be discussed further.
Thence, two samples were examined in details: the first one taken before sintering, the second one isolated after sintering at 150 °C.
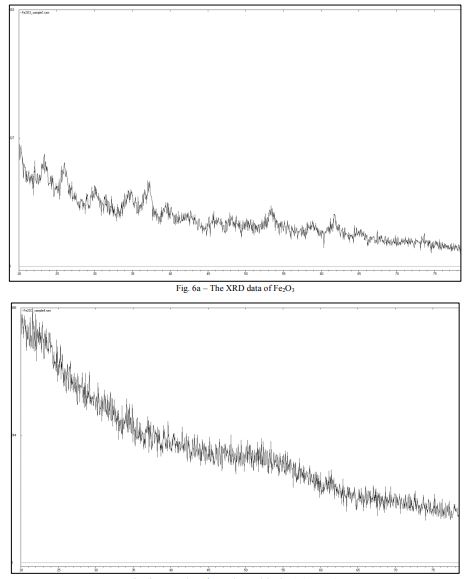
It can be seen from the XRD datathat the second sample had an amorphous structure with small inclusions of small crystallites 21(Fig. 6a), which did not quite satisfy our expectations.
The first sample that was taken before sintering represents, according to the XRD data, a completely amorphous substance (Fig. 6b) which was also evidenced by the EBSD analysis data. Details of the process are described in the EBSD section. It should be noted that the modified sol-gel method did not require high sintering temperatures to obtain an amorphous structure.
As an aluminum nitrate, iron nitric acid (III) was analyzed for the impurities using EPMA. The data showed that this compound had no impurities (Fig. 7, Table 7).
Table 8 – The EPMA analysis of the iron oxide calcined at 150 °C
| Spectrum Label | N, % | C, % | O, % | Fe, % | Total, % |
| S 1 | 0 | 0 | 78,41 | 21,59 | 100 |
| S 2 | 0 | 0 | 69,69 | 30,31 | 100 |
| S 3 | 0 | 0 | 73,67 | 26,33 | 100 |
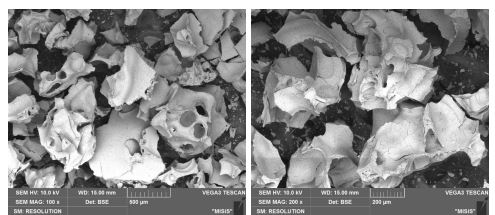
The EPMA data showed that the amorphous Fe2O3 had no impurities (Fig. 9, Table 9). The morphologies of both samples were flake-like.
Fig. 9 – The EPMA analysis of the amorphous Fe2O3
Table 9 – The EPMA analysis of the amorphous Fe2O3
| Spectrum Label | N, % | C, % | O, % | Fe, % | Total, % |
| S 1 | 0 | 0 | 64,49 | 35,51 | 100 |
| S 2 | 0 | 0 | 77,95 | 22,05 | 100 |
| S 3 | 0 | 0 | 67,51 | 32,49 | 100 |
Comparing these two samples, the sample taken after sintering at 150°C was not completely amorphous. This sample, as previously stated and as seen from the XRD data(Fig. 6a), had inclusions of small crystallites, in comparison with the sample taken after drying (Fig. 6b). It can be concluded that to obtain an amorphous structure of iron oxide (III), sintering can be carried out at low temperatures, which was fundamentally different from the synthesis of Al2O3. It can be seen that to achieve the desired result, just a long drying period of several hours was enough.
Thermal decomposition of the corresponding nitrate:
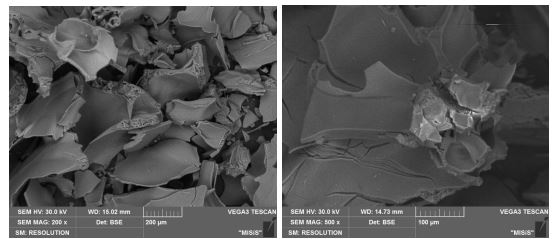
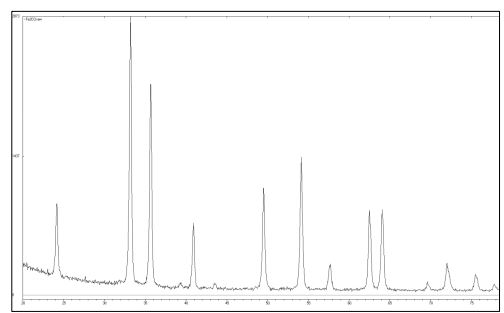
The XRD analysis datashowed that the compound isolated after the thermal decomposition of the iron salt using an electron oven at 100 °С had inclusions of large crystallites 21(Fig. 10). From the EPMA data, the substance was pure and had large crystals in its composition (Fig. 11, Table 10).
The residual amount of nitrogen and carbon compounds was not detected.
Fig. 10 – The XRD data of the iron oxide obtained by thermal decomposition
Fig. 11 – The EPMA analysis of the Fe2O3 obtained by thermal decomposition
Table 10 – The EPMA analysis of the Fe2O3 obtained by thermal decomposition
| Spectrum Label | N, % | C, % | O, % | Fe, % | Total, % |
| S 1 | 0 | 0 | 61,13 | 38,87 | 100 |
| S 2 | 0 | 0 | 60,71 | 39,29 | 100 |
| S 3 | 0 | 0 | 20,07 | 79,93 | 100 |
- EBSD
The objects of research were cylindrical samples, which were obtained by pressing at a pressure of 100 MPa powder of oxides Al2O3 and Fe2O3 with a diameter of 4-5 mm and a height of 7 mm with plane-parallel surfaces. The sections were prepared using the Struers Labopol-5 system. Further, the samples were pressed into a polystyrene polymer, the surface was ground on sandpaper of different grain sizes (220, 320, 800, 1200, 2400, 4000), then polished on a water-alcohol suspension of SiO2.
The samples were investigated using the method of automatic analysis of backscattered electron diffraction (EBSD) patterns on a TESCAN VEGA LMH scanning electron microscope with a LaB6 cathode (SEM) with an Oxford Instruments Advanced AZtecEnergy X-ray energy dispersive microanalysis system and an EBSD NordLysMax2 Oxford Instruments attachment with the AZtec version 3.5 software. Various databases were used to identify materials and phases: the Oxford Instruments database of pure elements and organic substances, the NIST database of phases and elements. Scanning was carried out with a step of 0.1 μm.
The essence of the method is: a sample tilted at an angle of 70° was placed in a microscope chamber. The electron beam was focused on the sample surface and interacts with the sample atoms. An angle of 70° allowed to increase the number of reflected electrons, because in this case, the region of interaction of the beam electrons with the sample will be located closer to the surface, which increased the possibility of electrons escaping from the sample. The backscattered electron detector captured these electrons, which provided information about the intensity of the backscattering of electrons in different areas of the sample surface. Some of the reflected electrons are diffracted from different areas of the crystal in accordance with the Wolfe-Bragg law (n×l=2d×sinq, where q - the angle between the incident electron of the beam and the plane from which it was reflected) and form the diffraction pattern of the Kikuchi line. A Kikuchi line is a pair of parallel lines caused by diffraction of reflected electrons from one specific area of the crystal lattice. Diffraction from all atomic areas set of Kikuchi lines (called the diffraction pattern) on the phosphor screen.
Automatic indexing used the Mean Angular Deviation (MAD), it meant the quality of the solution. The smaller the number, the better the match between the detected Kikuchi bands and the simulated phase lines. According to the accepted phase identification rules, an SDD value less than 1 indicates a good match. The work used the OMS coefficient <0.35.
In all samples, diffraction patterns (Kikuchi lines) and any textures were not detected, which indicates the absence of a crystal lattice and confirms amorphousness.
- Thermal stability of amorphous oxides
The temperatures of recrystallization (deamorphization) of the oxides depending on the heating rate were determined by differential scanning calorimetry to evaluate the thermal stability of the obtained amorphous oxides.
- Al2O3
Recrystallization (de-amorphization) temperature
DSC heating curves of amorphous Al2O3 obtained by the modified sol-gel method, measured at heating rates of 5, 10, 15, and 20 K/min, respectively, are shown in Figure 12. No other peaks associated with phase transformations on heating up to 1100 °C and on subsequent cooling were observed.
Fig. 15 – DSC heating curves of amorphous Fe2O3 obtained at different heating rates
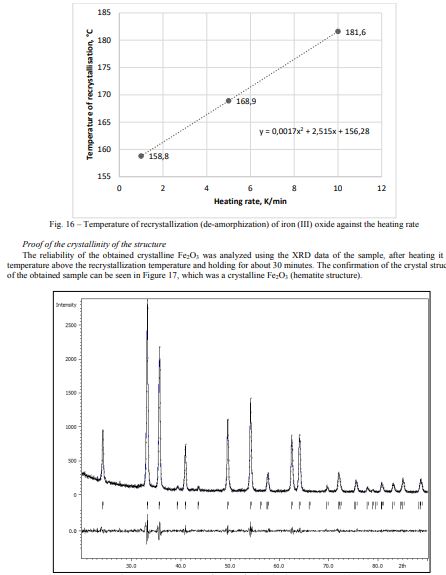
The dependence of the crystallization onset temperature on the heating rate was shown in Figure 16. The recrystallization temperature increased with the heating rate linearly.
Fig. 17 – XRD spectrum of Fe2O3 after heating in an oven over 200 °C
- The enthalpy of fusion (or crystallization)
This test method was applicable to solid samples in granular form and described the determination of the enthalpy (heat) of fusion (melting) and crystallization by differential scanning calorimetry (DSC) 22.
Using the thermal curve, can be constructed a baseline on the differential heat flow curve by connecting the two points at which the melting endotherm deviated from the baseline before and after the melt (Fig. 12 and Fig. 15). After this area can be integrated as a function of time to achieve the melting endothermic peak area in mJ 23.
The experimental calibration coefficient at the melting temperature of the standard reference material can be calculated as:
| E = (H·m)/(A) | (1) |
where:
E – calibration coefficient at the temperature of the melting endotherm,
H – enthalpy of fusion of the standard material, in J/g or (mJ/g),
m – mass of the standard, in g, and
A – melting endotherm peak area, in mJ.
| H = E·Ho/W | (2) |
where:
H – enthalpy of fusion (or crystallization) of the sample in kJ/mol,
W – mass of the specimen, mg,
E – Calibration constant from (1),
Ho – observed enthalpy of fusion (or crystallization), mJ.
- Al2O3
The standard reference material for aluminum oxide was Ag measured at 5 K/min. It was chosen based on the similarity between the melting point of silver and the recrystallization temperature of amorphous Al2O3.
According to (1), the calibration coefficient at the temperature of the melting endotherm can be calculated (Table 11):
Table 11 – The calibration coefficient at the temperature of the melting endotherm data of Al2O3
| E | H, mJ/g | m, g | A, mJ |
| 105 681,93 | 104,8e+6 24 | 0,01414 | 14,022 |
According to (2), the enthalpy of fusion (or crystallization) of the sample can be calculated (Table 12):
Table 12 – The enthalpy of fusion (or crystallization) data of Al2O3
| H, kJ/mol | E | Ho, mJ | W, mg |
| -15,02 | 105 683,93 | -26,944 | 19,33 |
The enthalpy of fusion (crystallization) of amorphous Al2O3, according to calculations, is -15.02 kJ/mol. For example, this value was studied earlier for the gamma modification by Qiyuan and is -26.78 ± 0.41 kJ/mol 25.
- Fe2O3
Similarly, the standard reference material for iron oxide (III) was In measured at 5 K/min. It was chosen based on the similarity between the melting point of indium and the recrystallization temperature of amorphous Fe2O3.
According to (1), the calibration coefficient at the temperature of the melting endotherm can be calculated (Table 13):
Table 13 – The calibration coefficient at the temperature of the melting endotherm data of Fe2O3
| E | H, mJ/g | m, g | A, mJ |
| 146 199,19 | 28,51e+6 26 | 0,03786 | 7,383 |
According to (2), the enthalpy of fusion (or crystallization) of the sample can be calculated (Table 14):
Table 14 – The enthalpy of fusion (or crystallization) data of Fe2O3
| H, kJ/mol | E | Ho, mJ | W, mg |
| -59,84 | 146 199,19 | -46,644 | 18,2 |
The enthalpy of fusion (crystallization) of amorphous Fe2O3, according to calculations, is -59.84 kJ/mol.
- Heat capacity
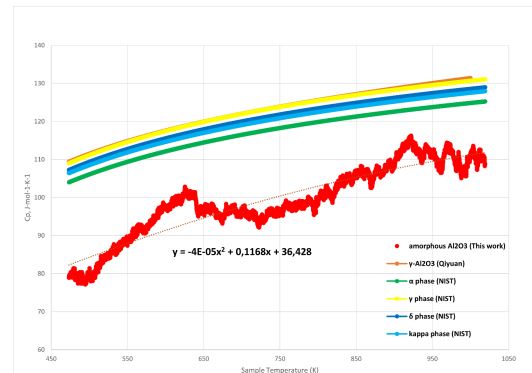
Based on the sensitivity of the device and the ability to measure heat capacity data only at high temperatures, amorphous alumina was selected as the object of this study. The data of the high-temperature heat capacity of amorphous alumina were compared with the literature data of the high-temperature heat capacity of gamma oxide by Qiyuan 25 and NIST data 27.
According to Qiyuan the relationship between the heat capacity of γ-Al2O3 and temperature could be expressed as
Cp = 115.25 + 19.53 × 10-3T - 33.81 × 105T-2 in the range 298.15 – 1000 K 25.
Or, based on the NIST data, the heat capacities of alumina phases can be calculated using
Cp = A + Bt + Ct2 + Dt3 + E/t2, where Cp – heat capacity (J/mol*K) and t – temperature (K)/1000, in a wide range of temperatures.
As expected, the plot of heat capacity versus temperature had an increasing character (Fig. 18).
On the plot of the heat capacity versus temperature was shown that the measurements were started in the range of 450 - 500 K. This phenomenon was associated with the low sensitivity of the device and the ability to display heat capacity data starting from about 473 K.
Fig. 18 – Heat capacity of amorphous Al2O3 compared to literature data
- Conclusion
In the present paper the amorphous structures of amphoteric oxides Al2O3 and Fe2O3 obtained the modified sol-gel method were investigated. It should be noted that the modified sol-gel method had a small number of stages, which also significantly reduced the synthesis time. It was shown that for Al2O3, in addition to the expected amorphous structure, the γ-modification can also be obtained 7. And as a result of obtaining Fe2O3, it turned out that it can be synthesized at significantly lower temperatures.
Thermal stability of the amorphous oxides was studied using the DSC method. It was found that amorphous Al2O3 is stable up to 790 – 810 °C, while amorphous Fe2O3 recrystallizes at ~ 160 – 180 °C. The dependence of the phase transition temperature on the heating rate of the samples was carried out, which had a linear character. Based on XRD data, the reliability of this experiment was proved by investigating the oxides obtained after the transition. As expected, the dependence of heat capacity on temperature, measured in the range 473 – 1050 K, had an increasing character.
| Финансирование Авторы выражают признательность за финансовую поддержку Министерства науки и высшего образования Российской Федерации в рамках Программы повышения конкурентоспособности НИТУ «МИСиС» (N◦◦2-2020-036), реализуемой Постановлением Правительства N 211 от 16 марта 2013 года. | Funding The authors gratefully acknowledge the financial support of the Ministry of Science and Higher Education of the Russian Federation in the framework of the Increase Competitiveness Program of NUST «MISiS» (N◦К2-2020-036), implemented by the Governmental Decree N 211 dated March 16, 2013. |
| Конфликт интересов Не указан. | Conflict of Interest None declared. |
Список литературы / References
- Enhancement of Ce/Cr Codopant Solubility and Chemical Homogeneity in TiO2 Nanoparticles through Sol–Gel versus Pechini Syntheses / W. Chen, et al. // Inorganic Chemistry. – 2018. – Vol. 57 (12). – pp. 7279–7289.
- The Chemistry of Metal Alkoxides / N. Turova, E. Turevskaya, V. Kessler et al. – Dordrecht: Kluwer Academic Publishers, 2002.
- Rabinovich V. Brief Chemical Handbook / V. Rabinovich, Z. Khavin. – Chemistry. 3rd ed., 1991.
- Kotov Y. Characteristics of aluminum oxide nanopowders obtained by the method of electric explosion of wire / Y. Kotov // Russian Nano Technologies. – 2007. – Vol. 2 (7–8). – pp. 109–115.
- "Corundum". Handbook of Mineralogy. III Halides, Hydroxides, Oxides / J. Anthony, R. Bideaux, K. Bladh et al. – US: Mineralogical Society of America, 1997.
- The Geology and Genesis of Gem Corundum Deposits, Gem Corundum / G. Giuliani, D. Ohnenstetter, A. Fallick, et al. – Research Gate: Mineralogical Association of Canada, 2014. – 37-38 p.
- Paglia G. Determination of the Structure of γ-Alumina using Empirical and First Principles Calculations Combined with Supporting Experiments / G. Paglia. – Curtin University of Technology, 2004.
- Levin I.Metastable Alumina Polymorphs: Crystal Structures and Transition Sequences / I. Levin, D. Brandon // Journal of the American Ceramic Society. – 1998. – Vol. 81 (8). – pp. 1995–2012.
- Kurt J. SDS of Iron (III) oxide / J. Kurt. – KJLC, 2014.
- Greedan J. Magnetic oxides, Encyclopedia of Inorganic chemistry / J. Greedan. – 1994.
- Elliott S. Medium-range structural order in covalent amorphous solids / S. Elliott // Nature. – 1991. – Vol. 354 (6353). – pp. 445–452.
- Chemical properties of inorganic substances / R. Lidin, V. Molochko, L. Andreeva. – Chemistry, 2000. 480 p.
- Knunyants I. Chemical encyclopedic dictionary Soviet Encyclopedia / I. Knunyants. – 1983. – 200 p.
- Cui H. Structure switch between α-Fe2O3, γ-Fe2O3 and Fe3O4 during the large scale and low temperature sol–gel synthesis of nearly monodispersed iron oxide nanoparticles / H. Cui, Y. Liu, W. Ren // Advanced Powder Technology. – 2013. – Vol. 24. – pp. 93-97.
- Ztec A. Oxford Instruments Nanotechnology Tools Limited / A. Ztec. – 2010-2015. – 860 p.
- Kraus W.POWDERCELL, Rev. 1.8a / W. Kraus, G. Nolze. – Federal Institute for Materials Research and Testing (BAM), 1996.
- National bureau of standards. Standard Material 720. Synthetic Sapphire (α-Al2O3). –
- Tetelbaum D.Properties of Al2O3: nc-Si nanostructures formed by ion implantation of silicon into sapphire and amorphous aluminum oxide films / D. Tetelbaum, A. Mikhailov, A. Belov // Solid State Physics. – 2009. – Vol. 51 (2). – pp. 385-392.
- Zhou R.Structures and transformation mechanisms of the η, γ and θ transition aluminas / R. Zhou, R. Snyder // Acta Crystallographica. – 1991. – Vol. B47. – pp. 617–630.
- Housecroft C.Chapter 22: d-block metal chemistry: the first-row elements / C. Housecroft, A. Sharpe. // Inorganic Chemistry. – 3rd ed. – 2008. – 716 p.
- Ultrafine Particles of Iron (III) Oxides by View of AFM – Novel Route for Study of Polymorphism in Nano-world / M. Vujtek, R. Zboril, R. Kubineket al. – Departments of Experimental Physics, Inorganic, and Physical Chemistry: Palacky University, 2014.
- Standard Test Method for Enthalpies of Fusion and Crystallization by Differential Scanning Calorimetry. – 2012.
- Standard Practice for Heat Flow Calibration of Differential Scanning Calorimeters. – 2014.
- Emsley J. The elements, 3rd edition / J. Emsley. – Oxford Press: Oxford, 1998.
- Investigation of the thermodynamic properties of γ-Al2O3 / C. Qiyuan, Z. Wenming, C. Xinmin et al. // Thermochimica Acta. – 1995. – Vol. 253. – pp. 33–39.
- Values from National Institute of Standards and Technology (NIST). USA.
- Chase M. W. Jr. NIST-JANAF Thermochemical Tables, Fourth Edition / M. W. Jr. Chase // J. Phys. Chem. Ref. Data. – 1998. – Monograph 9. – pp. 1-1951.