Sol-Gel Fabrication of Hydrated Vanadium (V) Oxide and Analysis of Its Xerogel Properties
Sol-Gel Fabrication of Hydrated Vanadium (V) Oxide and Analysis of Its Xerogel Properties
Abstract
This study investigates the physicochemical properties of a xerogel derived from a highly stable hydrated vanadium (V) oxide sol, employing a range of analytical techniques including X-ray diffraction (XRD), gas adsorption (ASAP), thermogravimetric/differential scanning calorimetry (TG/DSC), and infrared (IR) spectroscopy. It was observed that the specific surface area of the V2O5 xerogel increased after heat treatment at 400 °C, but subsequently decreased with further heating to temperatures of 600 °C, 650 °C, and 700 °C. The nitrogen adsorption-desorption isotherm of the V2O5 xerogel revealed the presence of mesoporous structures, with pore diameters ranging from 2 to 100 nm, predominantly between 10 and 50 nm. Additionally, the crystallite size of the V2O5 powder was found to increase with elevated heat treatment temperatures. Notably, the V2O5 phase remained stable after heat treatment up to 700 °C. The infrared spectroscopy results indicated variations in the intensity and position of the absorption bands for the V2O5 xerogel across different heat treatment temperatures (400 °C, 600 °C, 650 °C, and 700 °C). Furthermore, the crystallization, melting, and solidification temperatures of the V2O5 xerogel were also established.
1. Introduction
In recent years, vanadium (V) oxide (V2O5) has attracted significant attention due to its unique electrical, optical, and catalytic properties. This transition metal oxide exhibits excellent performance in various applications including energy storage (especially in vanadium redox flow batteries), catalysis, sensors, and as a pigment in ceramics and glass. The importance of V2O5 in technological advancements necessitates a deeper understanding of its structural properties and how these properties can be manipulated through various synthesis techniques , , . One promising approach to achieve this is through sol-gel fabrication, which enables the controlled synthesis of V2O5 with tunable properties.
The sol-gel method is a versatile and well-established technique that allows for the production of oxide materials at low temperatures. It involves the transition of a system from a high dispersion system 'sol' into a solid 'gel' phase, which can be further processed to obtain powders, thin films, or bulk materials. This method is particularly advantageous for synthesizing complex oxides, as it facilitates homogeneity at the molecular level, leading to materials with desirable characteristics. The sol-gel process typically consists of several stages, including hydrolysis, condensation, gelation, drying, and thermal treatment, each of which can significantly influence the final properties of the resultant material .
The xerogel phase, which is obtained after the drying of the gel, acts as a precursor to the final crystalline phases that form upon subsequent thermal treatment. The thermal treatment temperature, in particular, plays a critical role in determining the phase transitions, crystallinity, surface area, and morphology of the synthesized material, ultimately impacting its functional properties. Understanding how varying thermal treatment temperatures affect the properties of V2O5 xerogels is crucial for optimizing their performance for specific applications .
V2O5 exists in several structural forms, with the most stable being the orthorhombic phase, which is typically achieved through heat treatment . The transition from the amorphous or xerogel state to crystalline V2O5 involves several exothermic reactions that can be influenced by the thermal regime employed. This article aims to explore the sol-gel synthesis of V2O5, focusing on the analysis of the properties of powders derived from its xerogel at various thermal treatment temperatures. By systematically varying the temperature during the thermal treatment phase, we will investigate how different annealing conditions affect the structural, morphological, and compositional properties of the resulting V2O5 powders. In addition to assessing crystallinity and phase identification, a variety of analytical techniques will be employed to characterize the synthesized V2O5 powders. These techniques will provide insights into particle size and distribution, surface area, porosity, and thermal stability. The findings from this study will contribute to a more comprehensive understanding of the relationship between the synthesis conditions, particularly thermal treatment, and the resultant properties of V2O5 powders. This study is expected to facilitate the design of V2O5 materials with tailored properties to optimize their performance in various applications.
2. Research methods and principles
The following reagents were used in this study: vanadium (V) oxide of analytical grade (MRTU 6-09-6594-70) and hydrogen peroxide (H2O2) of special purity grade (GOST-177 88). The sol was synthesized as follows: distilled water was added to a round-bottom flask containing a calculated amount of V2O5 powder and was dispersed while stirring. The calculated volume of hydrogen peroxide solution was then added, and the mixture was boiled for 10 minutes. The final product obtained was a hydrated vanadium (V) oxide sol with a dark red color.
The morphology and sizes of the nanoparticles were analyzed using a transmission electron microscope (TEM) LIBRA 200 FE HR (Germany). The morphology of the powder was examined with a scanning electron microscope (SEM) JEOL 1610LV (Japan) at the Center of Collective Use of the D.I. Mendeleev University of Chemical Technology of Russia. The crystallographic structure of the sample was analyzed using powder X-ray diffraction (XRD) DX-2700BH with Cu-Kα radiation. The average crystallite size was calculated using the Selyakov-Scherrer equation.
where k is a coefficient taken equal to 0.94; λ = wavelength of X-ray radiation; ϴ is the Bragg angle; β = width of the reflection at half maximum.
Thermal analysis of the powder samples was conducted using TG-DSC research on the STA 449F1 Jupiter® synchronous thermal analysis unit from Netzsch, in an atmosphere of argon gas grade "5.5" (99.9995%). The specific surface area of the xerogel was analyzed using the low-temperature desorption method on a specific surface and porosity analyzer (Micromeritics, USA) at the Center for Collective Uses of the D.I. Mendeleev University of Chemical Technology of Russia.
3. Main results
The synthesis of V2O5.nH2O sol was conducted following the previously established procedure described above, utilizing a molar ratio of vanadium (V) oxide to hydrogen peroxide of [1]:[30]. The sol concentrations varied from 0.3 to 1.6 wt.%. At concentrations below 0.3 wt.%, the system primarily exhibited a solution with precipitate formation. Conversely, concentrations exceeding 1.6 wt.% resulted in the simultaneous formation of both sol and gel phases. The morphology and electron diffraction patterns of the V2O5.nH2O nanoparticles are illustrated in Figure 1. As depicted, the solid phase consists of nanorods with a thickness of approximately 2 nm, a width ranging from 15 to 25 nm, and a length of 0.3 to 0.8 μm, exhibiting a polycrystalline structure. The colloidal chemical properties of these sols, evaluated at varying concentrations, were thoroughly analyzed in a previous study . A summary of the colloidal chemical properties is presented in Table 1 . In addition to the general properties outlined in Table 1, the relationship between sol concentration and viscosity, as well as the pH dependence on the coagulation threshold, was investigated in the prior study. Notably, a marked increase in sol viscosity was observed at concentrations surpassing 1% by mass. Also indicated that the maximum coagulation threshold occurred at a pH range of approximately 2.45 to 2.75; deviations in either direction from this pH resulted in a decrease in the coagulation threshold due to reduced sol stability .
Table 1 - Some of the colloidal chemical properties of the hydrated Vanadium (V) oxide sol
№ | Properties | Values |
1 | The region of concentration of sol that keep the sol’s stability | 0.3 to 1.6 mass % |
2 | pH that keep the sol stability | 2.35 to 3.6 ±0.2 |
3 | Electro kinetic potential, mV | -26±1 |
4 | The maximum point of optical density, nm | 378 nm |
5 | The rapid coagulation threshold value | 0.03 mol/liter NaNO3 |
Note: based on [7]

Figure 1 - HRTEM image and electron diffraction pattern of V2O5.nH2O nanorods
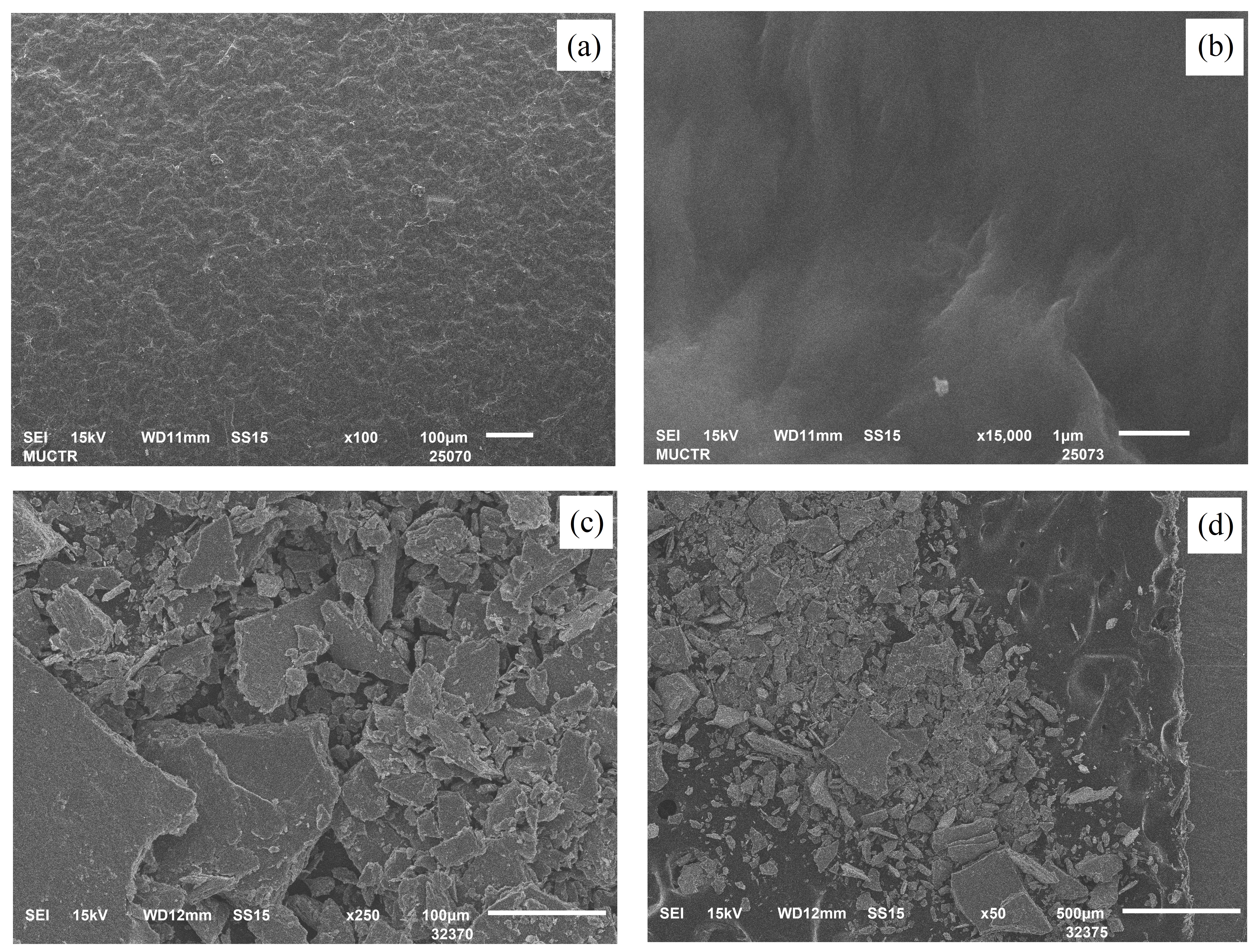
Figure 2 - SEM images of V2O5.nH2O xerogel film (a, b) on glass plate and (c, d) after removing from glass plate
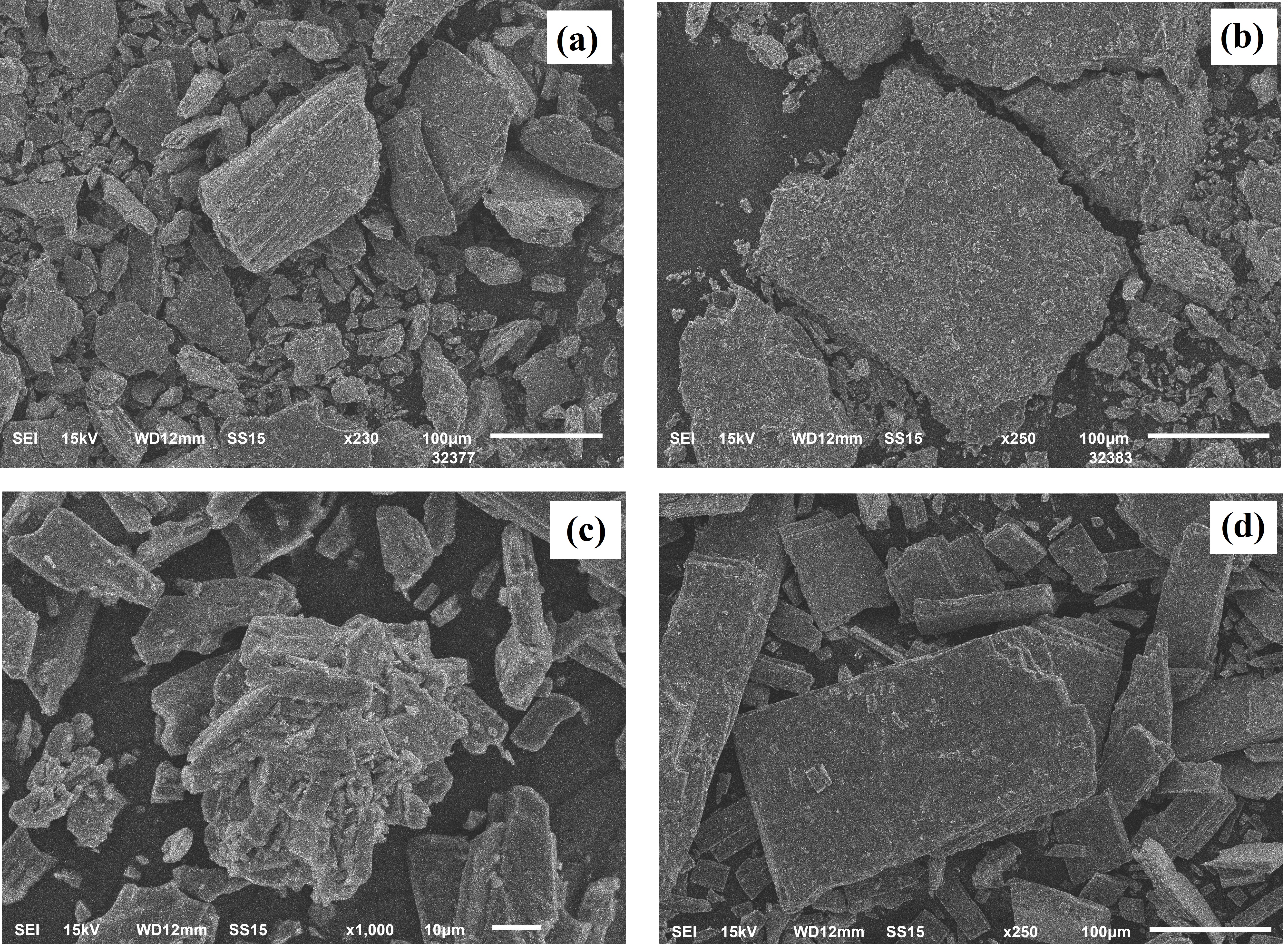
Figure 3 - SEM images of V2O5 powder after heat treatment at 400 °C (a), 600 °C (b), 650 °C (c) and 700 °C (d)
Cooling begins with the "e" effect, similar to the glass transition effect (inset in the figure). The effect begins at 655.2 °C, has an inflection at 653.7 °C, is in the middle at 653.5 °C, and ends at 651.8 °C. The change value is Cp=0.343 J/(g K). Almost immediately after the "e" effect, the "f" effect begins with a value of 180.3 J/g, corresponding to the solidification process. It begins at 640.9 °C, has a peak at 633.7 °C, and ends at 629.1 °C. No thermal effects were detected during further cooling.

Figure 4 - TG/DSC graph of xerogel V2O5.nH2O
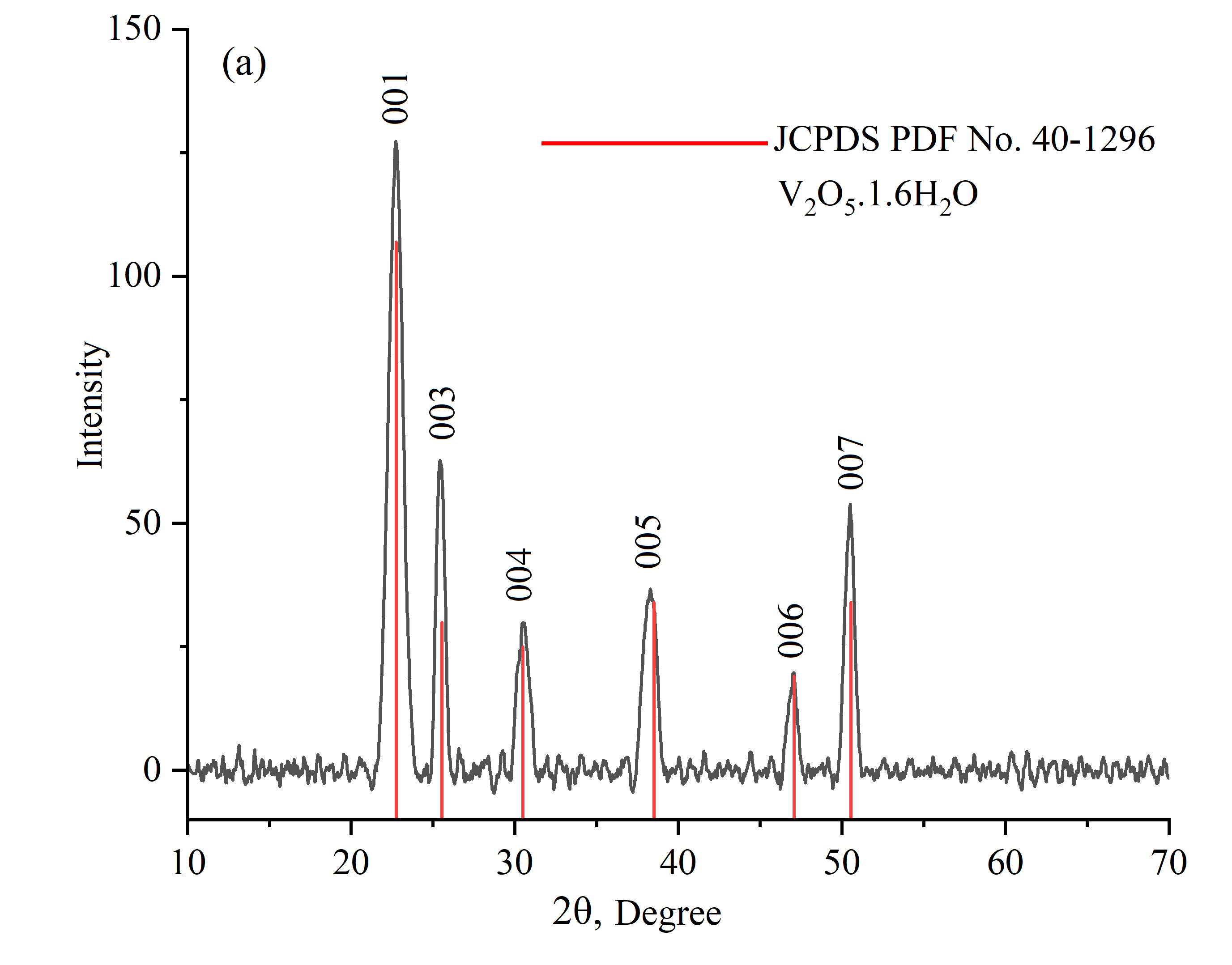
Figure 5 - X-ray diffraction patterns of V2O5.1.6H2O gel obtained after drying the sol at room temperature
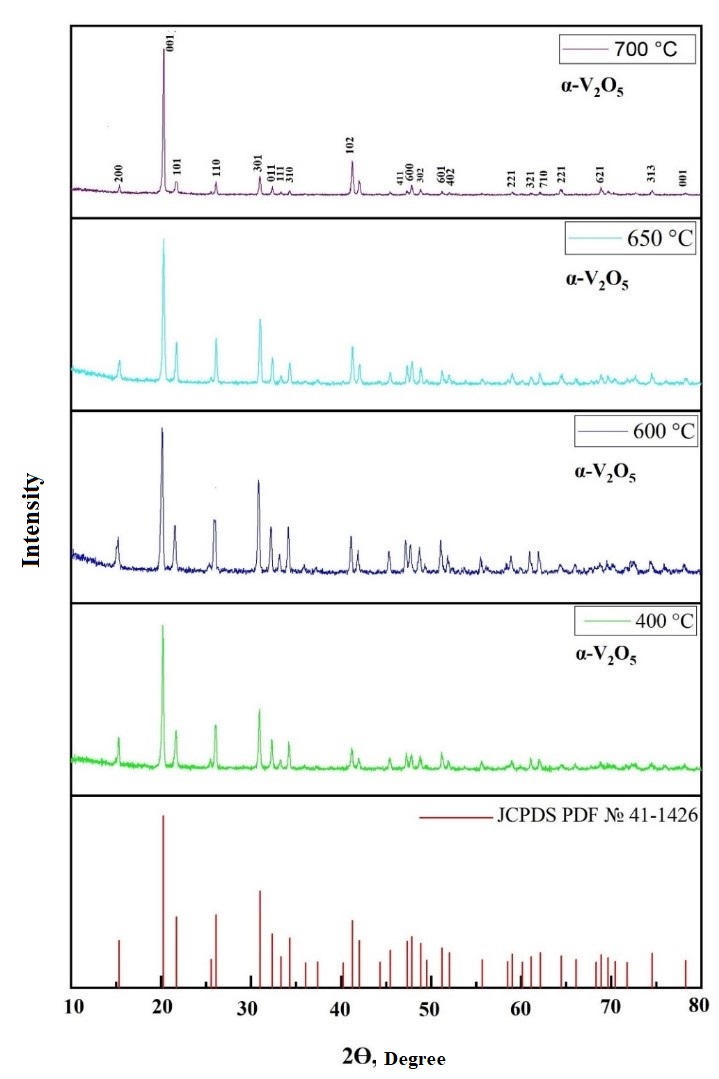
Figure 6 - X-ray diffraction patterns of V2O5 powder obtained after heat treatment of V2O5.1.6H2O xerogel at 400 °C / 600 °C / 650 °C and 700 °C one hour
Table 2 - Some crystal parameters of xerogel V2O5.1.6H2O and its powders
№ | Heat treatment temperature °С | Average crystallite size nm | Crystal lattice parameters | Crystal system | Phase | JCPDS PDF № | |
1 | Without thermal treatment | ~11 | а=3,5 Å; b=4,37 Å; c= 11,55 Å | α=90°; β =90°; γ=90° | Orthorhombic | V2O5.1,6H2O | 40-1296 |
2 | 400 | ~18 | а=11,516 Å; b=3,5656Å; c=4,3727 Å | α=90°; β =90°; γ=90° | α-V2O5 | 41-1426 | |
3 | 600 | ~18 | |||||
4 | 650 | ~18 | |||||
5 | 700 | ~19 | |||||
The specific surface area and porosity of the samples were analyzed through low-temperature nitrogen adsorption. The representative isotherms are depicted in Figure 7. Analysis of these isotherms revealed a type IV curve according to the Brunauer classification , indicative of polymolecular adsorption. Additionally, the presence of a hysteresis loop suggests capillary condensation within mesopores. This segment of the hysteresis loop corresponds to type H3 in the IUPAC classification , indicating the presence of slit-shaped pores. As seen in the images obtained through scanning electron microscopy (Figures 2 and 3), the xerogel exhibits aggregation form of nanorods, creating a layered structure with slit-like pores between the layers.
The adsorption data reveal that when the heat treatment temperature is increased to 400 °C, the specific surface area of the gel increases. This enhancement may be attributed to the removal of water molecules intercalated within the V2O5 layers, which reduces layer adhesion and consequently leads to the expansion of the V2O5 layers, increasing the surface area of the gel. However, as the heat treatment temperature is elevated further to 600, 650, and 700 °C, a decrease in specific surface area is observed. The specific surface area and porous characteristics of the xerogels subjected to various heat treatment temperatures are summarized in Table 3, as determined by the low-temperature nitrogen adsorption method.
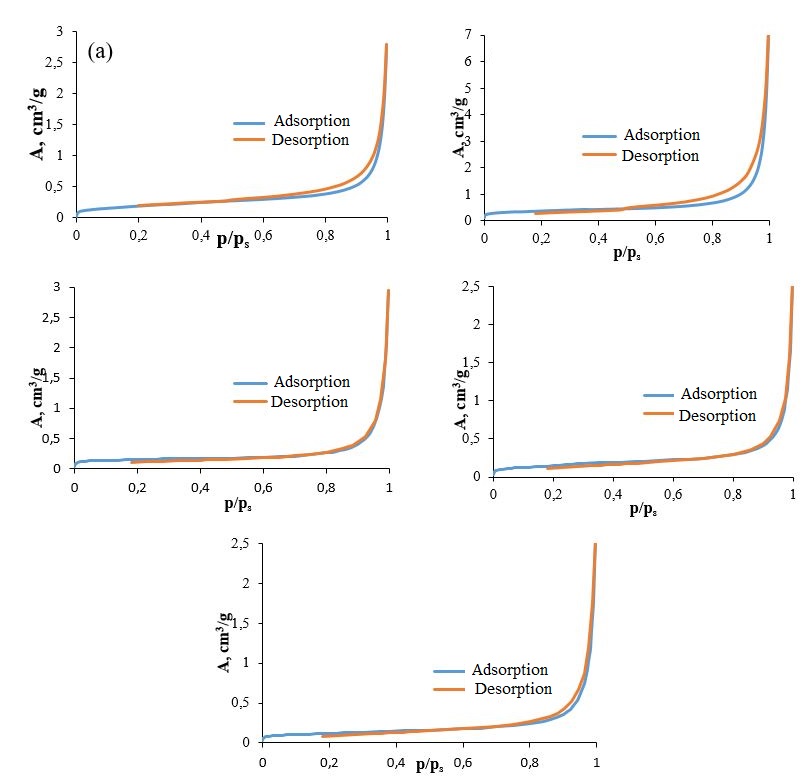
Figure 7 - Adsorption and desorption isotherms of N2 xerogel V2O5.1.6H2O (a) and samples obtained after heat treatment at 400 (b) /600 (c) /650 (d) /700 °C (d)
Upon subjecting the xerogel to heat treatment at 400 ºC, the IR spectrum reveals a notable decrease in the intensity of the bands associated with water, along with modifications in the intensity and positioning of the bands related to V–O vibrations. These changes reflect the structural alterations in the material as water is evaporated. As the heat treatment temperature is further increased, there is a reduction in the intensity of the V–O vibration bands, which can be attributed to a decrease in the surface area.
Table 3 - Porous characteristics of V2O5.1.6H2O xerogel at different heat treatment temperatures
№ | Temperature °C | BET Specific surface area, m2/g | BJH average pore diameter, nm | BJH desorption pore volume, cm3/g |
1 | Without thermal treatment | 0,6861 | 25,5216 | 0,004339 |
2 | 400 | 1,2731 | 30,1893 | 0,011036 |
3 | 600 | 0,5793 | 40,9484 | 0,004583 |
4 | 650 | 0,5456 | 30,907 | 0,003956 |
5 | 700 | 0,4237 | 30,2722 | 0,003942 |
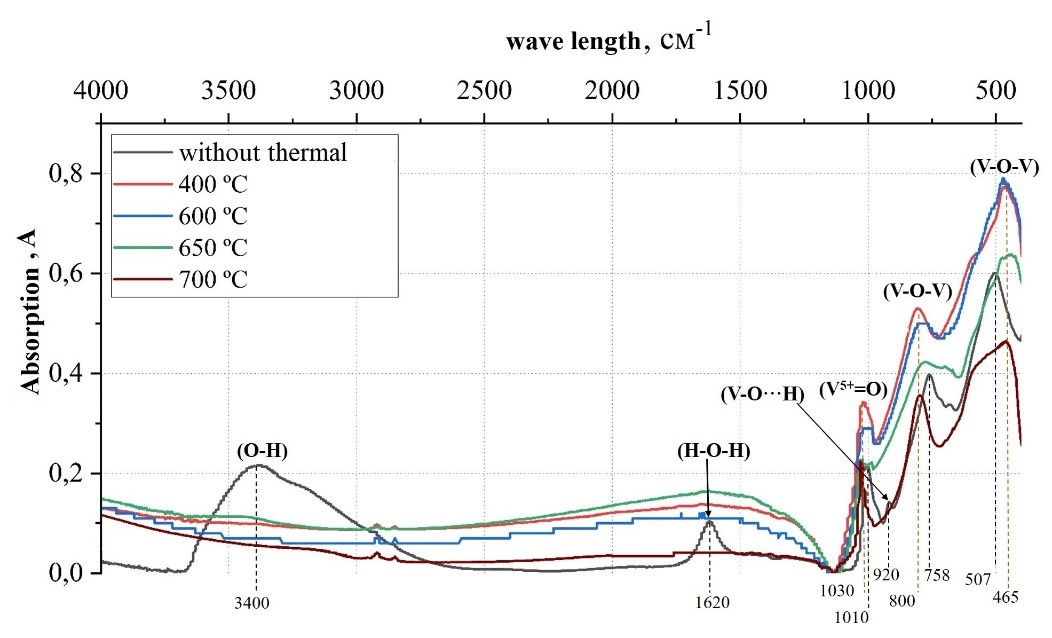
Figure 8 - Infrared spectra of V2O5.1.6H2O xerogel at different heat treatment temperatures
4. Discussion
In this study, we thoroughly examined the thermal influence on V2O5·1.6H2O xerogels derived from a stable hydrated V2O5 sol. The dispersion phase, characterized by the formation of nanorods, successfully created a thin film on the glass substrate. X-ray diffraction (XRD) analyses indicated that the xerogels exhibited an orthorhombic crystal structure. Notably, we observed that the crystallite size increased with higher thermal treatment temperatures. Specifically, when the thermal treatment reached 400 °C, the surface area of the xerogels increased, suggesting enhanced texture and porous characteristics. However, further thermal treatment beyond this temperature resulted in a decrease in surface area, indicating possible structural changes or densification that could adversely affect porosity. Additionally, thermogravimetric and differential thermal analyses (TG/DTA) provided critical insights into the melting, crystallization, and solidification points of the material, enhancing our understanding of the thermal stability and transformations occurring within the xerogels. Moreover, infrared (IR) spectroscopy effectively elucidated the molecular structure of vanadium species at various thermal treatment temperatures, contributing to a comprehensive understanding of the phase changes occurring in the material under thermal influence.
5. Conclusion
The investigation into the thermal influence on V2O5·1.6H2O xerogels reveals significant insights into their structural and physical properties. The findings highlight the importance of thermal treatment temperature on crystallite size and surface area, with optimal thermal conditions identified at 400 °C for maximizing surface area. However, further heating beyond this point may lead to undesired structural changes. The integration of XRD, TG/DTA, and IR spectroscopy has proven invaluable in mapping the thermal behavior and molecular characteristics of vanadium species within the xerogels. This comprehensive study lays the groundwork for future research on the application of V2O5 xerogels in various fields, including catalysis and energy storage, where understanding thermal stability and structural integrity is crucial.
