ANTICANCER AND TOXICITY ACTIVITIES OF NEW SYNTHESIZED COMPOUND
Гуля А. П.1, Тодераш И. К.2, Гудумак В. С.3, Цапков В. И.4, Гарбуз О. С.5, Рошков Е. В.6, Сардарь В. В.7, Тагадюк О. К.8
1ORCID 0000-0003-2010-7959, доктор химических наук, профессор, академик АН,
Кишиневский Государственный Университет, Кишинёв, Молдова
2ORCID 0000-0003-1599-838X, доктор биологических наук, профессор, академик АН,
Институт зоологии академии наук Молдовы
3ORCID 0000-0001-9773-1878, доктор медицинских наук, профессор,
Государственный университет медицины и фармации им. Н. Тестемицану, Кишинёв, Молдова
4ORCID 0000-0003-1732-3116, доктор химических наук, доцент,
Кишиневский Государственный Университет, Кишинёв, Молдова
5ORCID 0000-0001-8783-892X, аспирант;
Молдавский Государственный Университет, Кишинёв, Молдова
6ORCID 0000-0001-7565-2845, кандидат биологических наук,
Институт зоологии академии наук Молдовы
7ORCID 0000-0002-1047-9145, кандидат медицинских наук, 8ORCID 0000-0002-5503-8052, доктор медицинских наук,
7,8Государственный университет медицины и фармации им. Н. Тестемицану, Кишинёв, Молдова
ПРОТИВОРАКОВАЯ И ТОКСИЧЕСКАЯ АКТИВНОСТИ НОВОГО СИНТЕЗИРОВАННОГО ВЕЩЕСТВА
Аннотация
В данной работе представлен ряд сравнительных биологических исследований нового синтезированного вещества CMT-122.Антипролиферативная активность этого вещества тестировалась на двух клеточных линиях. Установлено, что CMT-122 проявляет цитотоксичность в отношении RD (рабдомиосаркома) с IC50 - 1,1±0.1 µmol/L и HeLa (аденокарцинома шейки матки) с IC50 - 8,3±2,0 µmol/L. Сравнительное изучение CMT-122 и доксорубицина в отношении раковых клеточных линий показало, что CMT-122 сильнее ингибирует пролиферацию раковых клеток, чем DOX. Дополнительный эксперимент, направленный на оценку цитотоксического эффекта с использованием нормальной клеточной линии MDCK (Madin Darby Canine Kidney), показал, что CMT-122 практически не ингибирует пролиферацию и не вызывает гибель этой линии клеток. Токсичность вещества определяли спектрофотометрическим биоанализом на тест-объектах Paramecium caudatum. Установлено, что LC50 для CMT-122 в 5 раз меньше, чем у DOX.
Ключевые слова: противораковая активность, токсичность.Gulea А. P.1, Toderas I. K.2, Gudumac V. S.3, Tapcov V. I.4, Garbuz О. S.5, Roscov E. V.6, Sardari V. V.7, Tagadiuc O.C.8
1ORCID 0000-0003-2010-7959, PhD in Chemistry, Professor, Academician ASM,
Moldova State University,Moldova,
2ORCID 0000-0003-1599-838X, PhD in Biology, Professor, Academician ASM,
Academy of Sciences of Moldova Institute of Zoology
3ORCID 0000-0001-9773-1878, MD, Professor,
State University of Medicine and Pharmacy “Nicolae Testemitanu”, Chisinau,
4ORCID 0000-0003-1732-3116, PhD in Chemistry, Associate professor,
Moldova State University, Chisinau, Moldova,
5ORCID 0000-0001-8783-892X, Postgraduate student,
Moldova State University, Chisinau, Moldova,
6ORCID 0000-0001-7565-2845, PhD in Biology,
Academy of Sciences of Moldova Institute of Zoology,
7ORCID 0000-0002-1047-9145, MD, 8ORCID 0000-0002-5503-8052, MD,
7,8 State University of Medicine and Pharmacy “Nicolae Testemitanu”, Chisinau, Moldova
ANTICANCER AND TOXICITY ACTIVITIES OF NEW SYNTHESIZED COMPOUND
Abstract
This work represents a series of comparative biological studies of the new synthesized compound CMT-122, exhibiting selective cytotoxicity.The antiproliferative effect of this compound was tested on two cell lines. It was established that CMT-122 exhibited cytotoxic activity against cell lines RD (rhabdomyosarcoma), HeLa (cervix carcinoma) with IC50 values of 1,1±0.1; 8,3±2,0 µmol/L, respectively. Comparative study between test compound and doxorubicin in regard to cancer cell lines was showed that CMT-122 exhibits stronger inhibitory activity on cancer cells proliferation than DOX. An additional experiment aiming on the evaluation of the citotoxic effect on MDCK (Madin Darby Canine Kidney) normal cells of line revealed that compound CMT-122 does not inhibit proliferation through induction of cell death. Toxicological testing method of compounds was performed by Paramecium caudatum colorimetric bioassay. It was founded, that the LC50 for compound CMT-122 is 5 times less than DOX.
Keywords: anticancer activity, toxicity.
Introduction
According to a recent report by the World Health Organization, there are now more than 10 million cases of cancer per year worldwide. Cancer refers to a diversity of diseases, characterized by the uncontrolled proliferation of cells into a different form, against the normal complement of the organism. The continuous proliferation of cancer cells develops into tumor tissues and may spread across to other organs. The principal need in the chemoprevention of cancer remains the discovery of new effective and safe agents, since the therapeutic application of antiproliferative drugs are restricted due to their toxic potentials, resistance and genotoxicity [1, P. 1659].
This work represents the research results of the new synthesized compound CMT-122 exhibiting selective cytotoxicity [2, P. 774], [3, P. 59]. The antiproliferative effect [4, P. 5189], [5, P. 78], [6, P. 650] of compound was determined using subsequent human cancer cells of lines: HeLa (cervix carcinoma) and RD (rhabdomyosarcoma).
Considering that drugs are primarily metabolized in the liver and excreted by the kidneys, renal impairment can ultimately affect the treatment outcome and toxicity. Based on this, we exploited MDCK (Madin Darby Canine Kidney) normal cells of line for selective cytotoxicity evaluation. The antiproliferative activity [7, P. 674] of compound CMT-122 was determined by alamar blue dye, which is one of the indicators of mitochondrial metabolic activity.
To estimate the results on the in vitro cytoxicity of the cancer and normal cell lines, the tested compound CMT-122 was compared to doxorubicin (DOX) as a positive control, which is used in the clinical management of a wide range of cancers [8, P. 806].
The direct toxic evaluation of o the tested compound CMT-122 was studied, by the colorimetric neutral red bioassay, using as test-objects the protozoan Paramecium caudatum, which is one of the most commonly used test-objects in laboratory research aimed at directly determining the toxicity of chemical compounds, which are used in toxicological medicine.
Experimental
Cell Culture
HeLa, RD and MDCK cells of line in this study were used. All cells of lines were grown cultured in T-75 cell culture flasks using Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12) supplemented with HEPES, L-Glutamine, 10% (FBS) fetal bovine serum and 100 U/ml of penicillin-streptomycin. Cells were maintained at 37oC in a humidified 2% CO2 atmosphere.
Alamar blue in vitro proliferation analysis
Cells viability was measured using the Alamar blue assay with each data point measured in triplicate. The absorbance was read by Hybrid reader H1 (Bio Tek) with 570 nm and 600 nm filters.
The percentage inhibition was calculated according to the formula:
Inhibition (%) = 100 - ((Abs570nmsmp. - Abs600nmsmp.) / (Abs570nmcon. - Abs600nmcon.) × 100).
Neutral red in vivo toxicity analysis
Direct toxic evaluation of compound CMT-122 was studied by colorimetric neutral red assay [9] of the quantification of the membrane permeability and lysosomal activity of Paramecium caudatum [10, P. 445]. The neutral red uptake assay provides a quantitative estimation of the number of viable cells in a culture.
Neutral red (3-Amino-7-dimethylamino-2-methylphenazine hydrochloride) was used for four hours, which is a weak cationic dye that easily penetrates the cell membrane and accumulates intracellularly in lysosomes, where it binds with anionic sites to the lysosomal matrix. The quantity of neutral red dye incorporated into cells was measured in 96-well plates by Hybrid reader H1 (Bio Tek), by spectrometry at 540 nm and 690 nm. All data about total toxicity activity are the averages of triplicate measurements.
Results and discussion
The antiproliferative activity of compound CMT-122 on two cell lines was tested using the Alamar blue method. Comparative study and concentration ranges identification of cytotoxic activity of CMT-122 and DOX in regard to RD, HeLa cancer cell lines and MDCK normal cell line are shown in Fig. 1.
It was found, that the tested compound CMT-122 exhibited in vitro cytotoxic activity against RD, HeLa lines, with IC50 values of 1,1±0,1; 8,3±2,0; µmol/L, respectively. In addition, compound CMT-122 showed low citotoxic activity against MDCK line, with IC50 values of ≥100 µmol/L. DOX, a positive control, exhibited cytotoxic activity against cell lines, RD, HeLa and MDCK, with IC50 values of 2,3±0,9; 10,0±4,0; 7±2,3 µmol/L, respectively.
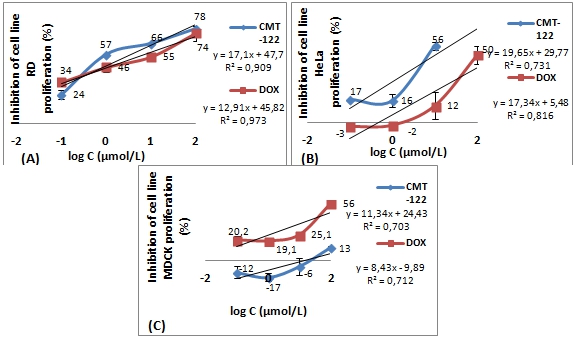
Fig. 1 – The effects of compound CMT-122 and DOX on the proliferation of several cells of lines after 24 hrs exposure: (A) inhibition of cell proliferation on RD line; (B) inhibition of cell proliferation on HeLa line; (C) inhibition of proliferation on MDCK line
Ultimately, our results have demonstrated that compound CMT-122 exhibits stronger inhibitory activity on RD, HeLa cancer lines proliferation than DOX. Concomitant was found, that the cytotoxic activity of compound CMT-122 on MDCK normal cells of line is significantly lower than that exerted on the cancer cells, and lower than that exerted by DOX.
The toxicity activity of the tested compound and DOX was performed by Paramecium bioassay. The effect of compound CMT-122 and DOX at a single concentration 100 µmol/L on the locomotor behavior of Paramecium caudatum was investigated for 30 min by inverted microscope (LOMO) with camera, and compared with control where organisms P. caudatum without treatment (Fig. 1). In culture with compound CMT-122 and DOX the following changes were observed: ciliates actively moved, almost did not form a cluster, there were single fixed specimens, also cellular volume initially increased followed by disintegration of protoplasm and internal membranes (Fig. 2.). The DOX was found more potent for lysis of Paramecium caudatum among compound CMT-122.
After 24 hrs, P. caudatum were in the bottom of wells. The light microscope revealed that most of the protozoa P. slowly moved. Some organisms in this period were motionless, contractile vacuoles were ruptured and their contents were thoroughly mixed up with protoplasm, appears as coagulation of proteins.
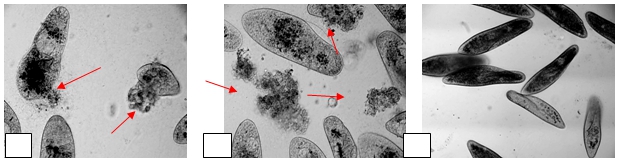
Fig. 2 – Effects of (A) compound CMT-122 and (B) DOX on Paramecium caudatum exposed the high concentration 100 µmol/L after 30 min; (C) organisms without treatment. Arrows indicating rupture of outer membrane
Percent of viability was determined after 24 hrs treatment for compound CMT-122 and DOX, it is graphically indicated in Fig. 3. It was founded, that the LC50 (lethal concentration) value is 4,9±1,5 µmol/L for CMT-122 and is 1±0,4 µmol/L for DOX.
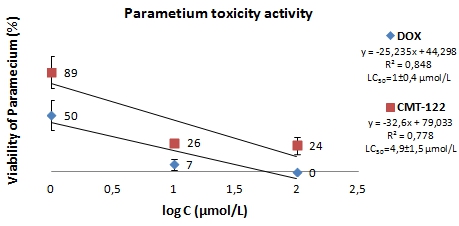
Fig. 3 – Effect of compound CMT-122 and DOX on population growth of Paramecium caudatum
Thus, our results have demonstrated that CMT-122 is lower toxicity than that exerted by doxorubicin.
Conclusion
In summary, these results suggest that the tested compound CMT-122 is of great interest due to their possibility for use as less toxic and more effective anticancer drug. The obtained data will serve as a basis to determine further investigations ways to elucidate pathogenic intimate and detailed mechanisms that can certainly be used to optimize and improve the effectiveness of the cancer treatment.
Список литературы / References
- Mohamed A Ismail. Anticancer, antioxidant activities, and DNA affinity of novel monocationic bithiophenes and analogues / A Ismail Mohamed, K Arafa Reem, M Youssef Magdy, M El-Sayed Wael // Drug Design, Development and Therapy – 2014. – P. 1659–1672.
- Rosu T. Metal-based biologically active agents: Synthesis, characterization, antibacterial and antileukemia activity evaluation of Cu(II), V(IV) and Ni(II) complexes with antipyrine-derived compounds / T. Rosu, M. Negoiu, S. Pasculescu, E. Pahontu, D. Poirier, A. Gulea // European Journal of Medicinal Chemistry. – 2010 – № 45. – P. 774–781.
- Gulea A. Synthesis and antileukaemia activity of N-(2,4-dimethylphenyl)hydrazine carbothioamide and its azomethine derivatives / A. Gulea, A. Sargun, A. Barbara, A. Jalba // Buletinul ASM. Seria Stiintele vietii. – 2012. – № 318 (3). – P. 59-66.
- Dilovic I. Novel thiosemicarbazone derivatives as potential antitumor agents: Synthesis, physicochemical and structural properties, DNA interactions and antiproliferative activity / I. Dilovic, M. Rubcic, V. Vrdoljak // Bioorg. Med. Chem. – 2008. – № – P. 5189–5198.
- Anoopkumar-Dukie S. Resazurin assay of radiation response in cultured cells / S. Anoopkumar-Dukie, JB Carey, T. Conere // British Journal of Radiology. – 2005. – P. 78-86.
- Pahontu E. Synthesis, Caracterization, Antimicrobial and antiproliferative Activity Evaluation of Cu(II), Co(II), Zn(II), Ni(II) and Pt(II) Complexes with Isoniazid-Derived Compound / E. Pahontu, D. Ilies, S. Shova, C. Oprean, V. Paunescu, O. Tudorel Olaru, F. Stefan Radulescu, A. Gulea // Molecules. – 2017. – № 22. – P. 650.
- Pahontu E. Synthesis and Characterization of Novel Cu(II), Pd(II) and Pt(II) Complexes with 8-Ethyl-2-hydroxytricyclo(7.3.1.02,7)tridecan-13-onethiosemicarbazone: Antimicrobial and in Vitro Antiproliferative Activity / E. Pahontu, Paraschivescu, D. Ilies, D. Poirier, C. Oprean, V. Paunescu, A. Gulea // Molecules. - 2016. – № 21. – P. 674.
- Gulea A. In vitro antileukemia, antibacterial and antifungal activities of some 3d metal complexes: Chemical synthesis and structure - activity relationships / A. Gulea, D. Poirier, J. Roy, V. Stavila // Journal of Enzyme Inhibition and Medicinal Chemistry. – 2008. – № 23 (6). – P. 806-818.
- Toderas I. Express method for testing toxic substances on the Paramecium caudatum culture using the Red Neutral Dye / I. Toderas, A. Gulea, V. Gudumac, E. Roscov, O. Garbuz // Patent application – № S2017 0067.
- Kryuchkova M. Evaluation of toxicity of nanoclays and graphene oxide in vivo: a Paramecium caudatum study / M. Kryuchkova, A. Danilushkina, Y. Lvovab // The Royal Society of Chemistry. Nano. – 2016 - № – P. 442 -452.
