Homeostasis in metabolically healthy obesity
Homeostasis in metabolically healthy obesity
Abstract
Background: Two complementary viewpoints determine clinicians’ close attention to the phenomenon of metabolically healthy obesity. Under the influence of additional risk factors, the studied obesity phenotype might become an intermediate stage of transition to metabolic ill-health. If this assumption is true, such a phenotype is an object of search for triggers for preventing the development of the disease. The research aims to assess changes in the structures of regulation of metabolic processes in healthy volunteers differing in body weight and hormonal status under conditions of a standardized meal.
Methods: We conducted a cross-sectional examination of healthy volunteers aged 18-43 years. Using generally accepted laboratory research methods, biochemical blood testing was done an hour after a standardized meal. In addition, the content of insulin and cortisol in the blood plasma was determined. When assessing the response of metabolic status or health status, a standardized meal was used as a stimulus. The meal allowed us to identify the maximum involvement of metabolic regulators or the “tension” of the system.
Results: The analysis of the characteristics of the strength of the statistical relationship between the analyzed values clearly demonstrated that increasing body weight and hyperinsulinemia gradually involve a “higher” level of regulation of metabolic processes in healthy volunteers.
Conclusion: With metabolically healthy obesity and associated hyperinsulinemia, a higher neurohormonal level is involved in metabolic control, significantly complicating regulation and increasing the risk of structural disturbances of homeostasis up to its transition to a metabolically unfavorable status.
1. Introduction
Recently, much attention has been paid to the concept of metabolically healthy obesity (MHO), which is understood as the presence of excess body weight in a person without any significant metabolic disorders (dyslipidemia, impaired insulin resistance, etc.) . A number of prospective studies have demonstrated that in 30% to 40% of cases, MHO may progress to a metabolically unhealthy obesity within 6 years . These data suggest that MHO might be a transient condition leading to a metabolically unhealthy status in the presence of other risk factors . Of great interest is metabolic regulation in people with MHO. Under normal conditions, metabolism is self-regulating and completely self-sufficient. The hierarchy of regulation of metabolic processes is carried out at several interconnected levels. Cellular metabolism is implemented through various compounds that are secreted into the intercellular space and interact with receptors of either nearby cells or the same cell. The next level is formed by systems that, under normal conditions, ensure the constancy of biochemical parameters of cells and organs. The organism level maintains the constancy of the internal environment of the organism through neurohumoral regulation and metabolism . Each subsequent level coordinates the work at the previous levels, coming into action when the external environment changes. A regular meal that covers a person’s basic needs is an example of a change in the external environment. Regulation of metabolism ensures the stability of homeostasis due to the balance of the influx of substances and energy into the cell, their storage or removal of decay products. Does the hierarchy of metabolic regulation change in individuals with MHO? It is a question that may underlie this phenomenon and, accordingly, methods for preventing its transition to a metabolically unfavorable status. The research work aimed to evaluate changes in the regulation structures of metabolic processes in healthy volunteers with different body weights and hormonal status under a standardized meal.
2. Research methods and principles
A single-center, interventional, cross-sectional study was carried out in the clinic of the Goldberg Research Institute of Pharmacology and Regenerative Medicine of Tomsk National Research Medical Center (TNIMC) of the Russian Academy of Sciences, Tomsk, Russia. The study included 34 healthy volunteers aged 18 to 43 years, including 19 men and 15 women.
Inclusion Criteria: healthy Caucasian male and female volunteers; non-smokers (last smoking episode was 6 months before the study); with the verified diagnosis “healthy” according to medical history, standard clinical, laboratory and instrumental examination; not obese, i.e. body mass index (BMI) within the range of 18.5 to 30.0 kg/m2.
Exclusion Criteria: the need for or use of medications; the subject’s decision to withdraw from the study or the subject’s refusal to cooperate with the research team; the subject’s failure to comply with the prescribed study regimen and restrictions; a positive pregnancy test in female subjects.
All volunteers underwent a metabolic activity study and hormonal status assessment one hour after the standardized meal intake.
The examination included an analysis of complaints, medical history and clinical examination. During the physical examination, a general examination was performed with measurement of height (m), body weight (kg); BMI was calculated using the formula: body weight (kg)/height (m)2. Using standard laboratory methods, biochemical blood testing was done one hour after the standardized meal of 500 kcal. In blood plasma, insulin and cortisol levels were determined using commercial enzyme immunoassay kits. All analysis procedures were carried out according to the instructions included with the kits, using a Stat Fax 2100 microplate reader (AWARENESS TECHNOLOGY Inc., USA). The insulin level was measured by the ELISA method using a standard reagent kit from DRG International, Inc. (Germany). Insulin and cortisol contents were expressed in μU/ml and μg/dl, respectively.
Variation statistics methods were used to process the results. Statistical analysis was carried out using Statistica 13.0 (TibCo, USA). Quantitative indicators were expressed as mean ± standard error of the mean. To compare the correlation coefficient for parametric data, we used the Pearson correlation coefficient (R) with the Yates correction and the likelihood correction. For nonparametric data, Spearman’s rank correlation coefficient was used (ρ). The analysis took into account only connections with a correlation coefficient R>0.6, characterizing a measure of strong linear dependence between characteristics and reflecting the rigidity of the relationship between the analyzed parameters.
The Local Ethics Committee of the Goldberg Research Institute of Pharmacology and Regenerative Medicine of TNIMC decided that this scientific work complies with the ethical standards of good clinical practice and can be carried out at the clinic of the Goldberg Research Institute of Pharmacology and Regenerative Medicine, TNIMC (Minutes No12 dated December 28, 2022). In accordance with the Declaration of Helsinki of the World Medical Association, each participant signed an informed consent for the examination approved by the Local Ethics Committee.
3. Main results
This article includes the results of a cross-sectional study of healthy volunteers who were not obese. Biochemical and hormonal parameters were studied an hour after a standardized meal of 500 kcal. It is believed that a pronounced increase in insulin occurs at 10-15 minutes after a meal in healthy individuals. Insulin helps compensate for the resulting hyperglycemia. However, normal glucose and insulin values are usually recorded 60 minutes later [5]. The obtained results did not meet our expectations: 10 volunteers had elevated glucose levels, up to a maximum of 8.3 mmol/l; in 14 people, insulin levels ranged from 25 to 85.5 µU/ml. It was not possible to use the data obtained to diagnose insulin resistance, since its assessment is carried out using calculated indices based on the ratio of fasting (basal) values of immunoreactive insulin and fasting plasma glucose. To understand the results obtained and analyze data, the volunteers were divided into 2 groups according to body mass index (BMI). Group 1 consisted of 13 people with normal body weight (BMI from 18.50 to 24.99 kg/m2). Group 2 included 21 volunteers with excess body weight (BMI > 25-29.99 kg/m2). Based on the results of the hormonal examination, we identified another group of volunteers with excess body weight and deviations in insulin levels from the reference values (Table 1).
Table 1 - Results of examination of volunteers depending on body mass index and insulin level
Indicator | All volunteers (n=34), (M±m) | Volunteers with a BMI from 18.50 to 24.99 kg/m2 (n=13), (M±m) | Volunteers with a BMI from 24.99 to 29.99 kg/m2 (n=21), (M±m) | Volunteers with a BMI from 24.99 to 29.99 kg/m2 and insulin level >25 µU/ml (n=10), (M±m) |
Age, years | 30.76±6.5 | 29.24±6.6 | 33.42±5.62 | 34.17±3.87 |
Height, cm | 172.61±8.37 | 170.86±7.02 | 175.67±9.92 | 178±10.33 |
Weight, kg | 72.64±14.19 | 65±7.87 | 86.0±12.92 | 86.83±14.37 |
BMI, kg/m2 | 24.21±3.2 | 22.21±1.73 | 27.72±1.78 | 27.2±1.7 |
Insulin, µU/ml | 27.48±19.51 | 24.66±17.16 | 32.4±23.02 | 50.62±18.74 |
Cortisol, mcg/dl | 6.44±2.71 | 6.46±3.08 | 6.42±2.04 | 6.4±2.91 |
Glucose, mmol/l | 5.72±1.02 | 5.63±0.89 | 5.86±1.26 | 6.53±1.53 |
Total cholesterol, mmol/l | 4.13±0.89 | 3.95±0.69 | 4.45±1.12 | 4.02±0.99 |
Triglycerides, mmol/l | 1.43±0.86 | 1.11±0.58 | 1.99±1.0 | 1.93±0.99 |
Aspartate aminotransferase, U/l | 17.18±7.36 | 15.92±3.91 | 19.38±11.03 | 15.88±1.86 |
Alanine aminotransferase, U/l | 15.84±9.2 | 14.11±7.76 | 18.86±11.01 | 15.87±6.46 |
Total protein, g/l | 65.12±3.04 | 65.54±3.19 | 64.38±2.73 | 63.33±2.28 |
Total bilirubin, µmol/l | 9.56±6.22 | 10.6±6.97 | 7.74±4.31 | 8.65±5.82 |
Urea, mmol/l | 3.55±0.69 | 3.43±0.73 | 3.75±0.61 | 3.63±0.76 |
Uric acid, µmol/l | 268.48±58.85 | 252.67±49.18 | 296.17±66.07 | 280.67±43.01 |
Creatinine, µmol/l | 67.55±11.11 | 65.29±10.67 | 71.5±11.2 | 75.33±8.59 |
Alkaline phosphatase, U/l | 55.21±20.4 | 53.66±23.79 | 57.93±12.98 | 60.52±13.4 |
GGTP, U/l | 18.13±10.65 | 15.97±9.18 | 21.92±12.33 | 22.37±15.75 |
Potassium, mmol/l | 4.39±0.31 | 4.43±0.34 | 4.33±0.25 | 4.27±0.19 |
4. Discussion
In each of the three subgroups of healthy volunteers, data analysis was carried out to assess indicators characterizing the strength of the statistical relationship between any two values.
In Group 1, 8 positive correlations were identified (R>0.6, Fig. 1). All relationships can be explained from the point of view of normal physiology. The relationship between triglyceride levels and screening markers of liver function is clear. The biological role of fats in the body – ensuring plastic and energy metabolism – is unthinkable without the metabolic function of liver enzymes. Under normal physiological conditions, blood glucose concentrations are invariably related to insulin levels. When food enters the body, there is a rise in blood glucose levels, which raises insulin secretion by the pancreatic β-cells. The described correlations in this group of volunteers reflect precise targeted control. Processes in the internal environment of the body trigger a strictly defined specific need for a certain physiological effect.
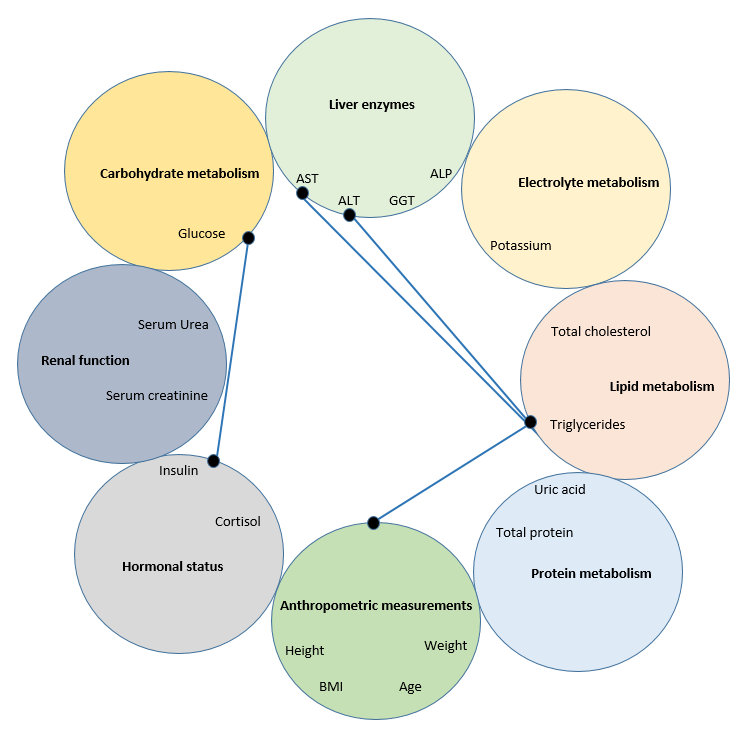
Figure 1 - Correlations (R>0.6) between indicators in healthy volunteers with a BMI of 18.5-24.9 kg/m2
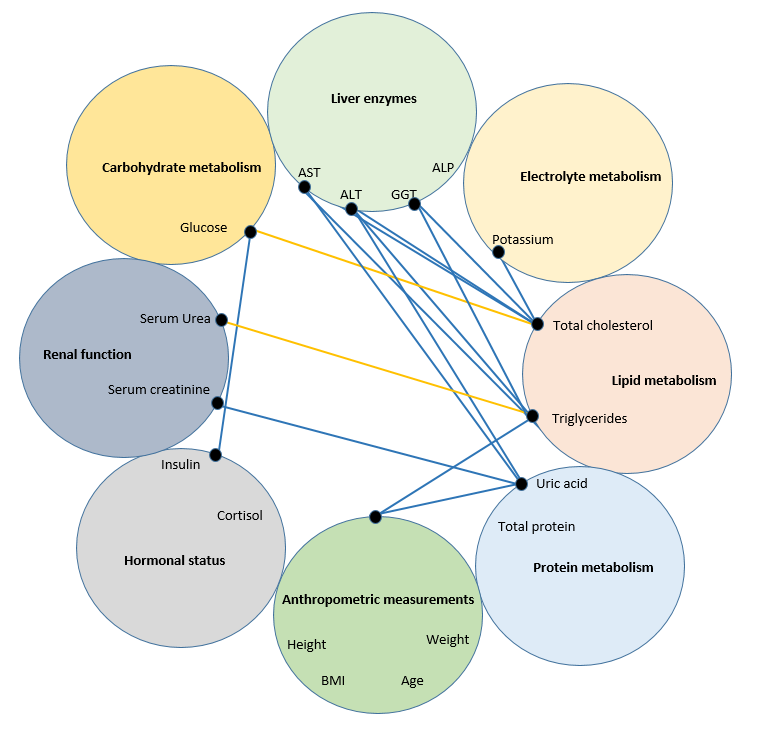
Figure 2 - Correlations (R>0.6) between indicators in healthy volunteers with a BMI of 24.9-29.9 kg/m2
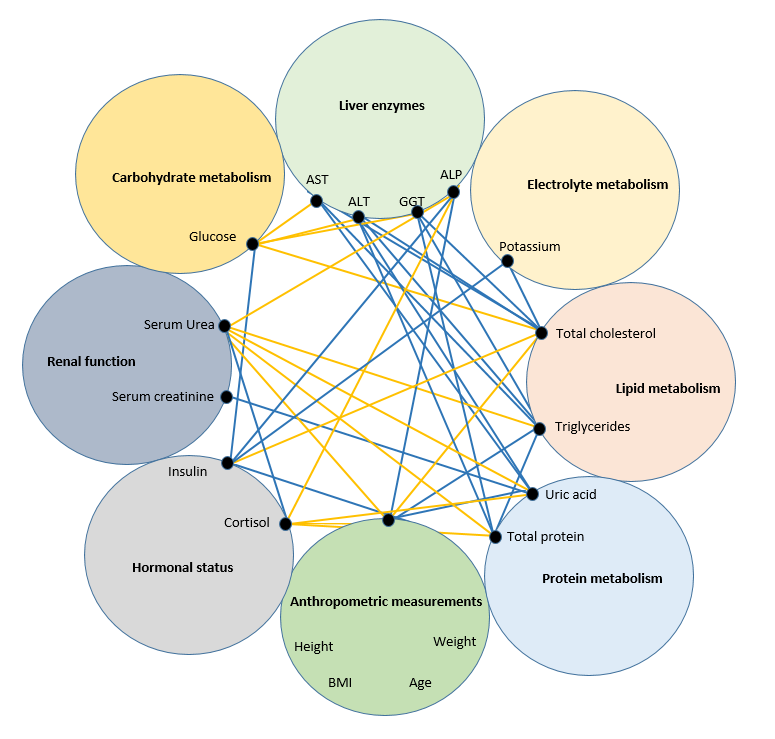
Figure 3 - Correlations (R>0.6) between indicators in healthy volunteers with a BMI of 24.9-29.9 kg/m2 and insulin levels >25 µU/ml
5. Conclusion
Of particular interest is the emerging integration of cortisol in the regulation of metabolic processes. Of course, maintaining homeostasis requires a complex interaction of a number of organs and systems of the body, including the neuroendocrine . The demonstrated manifestation of neurohormonal regulation characterizes its ability to simultaneously influence a number of different types of cells in the body and reflects the degree of tension of the regulatory systems necessary to maintain homeostasis, and determines the current functional state of the body with its physiological reactions focused on long-term effects.
