Monitoring of the Apixaban’s pharmacodynamics in patients with non-valvular atrial fibrillation
Monitoring of the Apixaban’s pharmacodynamics in patients with non-valvular atrial fibrillation
Abstract
The efficacy of Apixaban was studied in 11 patients with atrial fibrillation of non-valvular etiology based on the results of hemostatic potential monitoring by low-frequency piezothromboelastography and determination of anti-Xa factor activity under conditions of drug pharmacokinetics assessment by a validated HPLC-MS/MS method. It was shown that 72-hour pharmacokinetics of apixaban at a dosage of 5 mg. Twice a day has a sinusoidal character with Cmax at 4, 16, 28, 40, 52 and 64 hours of monitoring, and Cmin at 12, 24, 36, 48, 60 and 72 hours. When monitoring the clinical efficacy of apixaban based on the results of determining the activity of the anti-Xa factor, it was shown that it was possible to evaluate the pharmacodynamics of the drug only at its plasma concentrations exceeding 100 ng/ml. Monitoring the state and changes in the hemostatic potential during apixaban administration, performed by the method of low-frequency piezothromboelastography, demonstrated its ability to determine the pharmacodynamic effects of the drug at its plasma concentration within 45 ng/ml. It is noteworthy that the hypocoagulation status of the hemostatic potential achieved at the 2nd hour, in contrast to the pronounced changes in the plasma concentration of apixaban, does not undergo pronounced deviations at the stages of observation. This indirectly confirms the sufficient effectiveness of the selected dosage of the anticoagulant and the discreteness of its administration.
1. Introduction
Atrial fibrillation (AF) has been and remains one of the leading causes of fatal complications of cardiovascular diseases . In this regard, AF of non-valvular etiology (AFNE) is of particular interest, which is positioned as a disease with an increased risk of developing ischemic stroke and as a condition with its quite feasible prevention , . It is also natural that in the strategy for treating AFNE, a significant role is given to the prevention of thromboembolic and hemorrhagic complications , . It is for these reasons that AFNE, as a condition with mandatory drug antithrombotic accompaniment, can act as a convenient model for monitoring their pharmacodynamics, when apixaban is used. One of the main monitoring tests is the performance of the anti-Xa factor , , , , . As an adequate replacement for the “gold standard” of inspection the status system of the hemostasis in urgent clinical experience practice, that was relating to the observation and treatment of actual patients as well as to theoretical or laboratory researches – thromboelastography, a more sensitive worldwide experience for evaluating the hemostatic potential – low-frequency piezothromboelastography – is of particular attentiveness , , . In this instance, significant help in evaluating the clinical potency of apixaban and determining the sensitivity of that monitoring methods can be assured by details on the branch of mechanics соncentration of the NOAK in the blood founded on the influence of truncated pharmacokinetics. We used a similar approach in our working that was dedicated to watch the potency of dabigatran and rivaroxaban , .
The purpose of the study was to demonstrate the potency and sensitivity of the 5 mg dose of apixaban as a pharmacodynamic test monitoring option in the prevention of thrombotic sequelae in a group of AFNE patients.
2. Research methods and principles
The analysis work involved 11 patients with confirmed AFNE, men aged 35 to 56 years. People from this group were in signature agreement in conformity as required by the Helsinki statement, and the plan was approved by the local ethics committee.
Prior to the study, a catheter was inserted into a vein for the first 24 hours and blood was collected in 6 ml vacutainers to estimate plasma drug concentrations. A sample of blood was collected: initially, 10-15 minutes earlier than receipts of 5 mg of apixaban, and after 1, 2, 4, 6, and 12 hours, when the patient received another 5 mg of the medicament. Blood samples were then taken at 13, 14, 16, 18, and 24 hours of supervision, after whichever the catheter was removed. Twice a day, under the supervision of the medi, blood was collected with a syringe from the cubital vein at designated time intervals up to and including the third day. Plasma of blood was isolated by centrifugation at 300 rpm for 30 min with cooling, after completed manipulating it sent to a cryotube and instantly as a result of extreme cold frozen in dry ice. Then, examples were transported to the research laboratory for analysis of the anticoagulant concentration. The determination of POAK concentrations in the examples was performed by HPLC-MS/MS method as claimed according to the teaching . A quantitative test for determining the state of the blood aggregate state regulation system was supported by monitoring the activity of the anti-Xa factor using TS-POAK-control kits (manufactured by Technologiya-Standart LLC) on a Technology Solution automatic coagulometer, in which the necessary reagents were mixed with the analyzed patient samples, after which changes in optical density were recorded and the quantitative content of the POAK in the test sample was calculated . At the date intervals designated for evaluating the pharmacokinetics. In parallel (before receipts of the drug, after 2, 4, 6 and 12 hours of monitoring), the LFPTEG curve was recorded on an ARP-01M Mednord thromboelastograph. Native blood in quantity 0.45 ml was collected from a vein in the forearm of the hand without relevanting a wisp. Furthermore, the time between the collection of blood from the patient and its placement in the instrument's cuvette didn't exceed 20 seconds , .
Main idicators of the LFPTEG graph was performed by:
t1 – the period from the start of the research until the minimum LFPTEG amplitude is reached – A1;
t3 – the point of turning blood into a gel-like mass (HP) in min;
t5 – period of achievement maximum LFPTEG amplitude (A5);
ICD – the intensity of the coagulation drive, characterizing mainly the proteolytic stage of hemocoagulation;
TAC – the thrombin activity constant, as an intensity of the proteolytic level of formation of blood fibrin;
MA – the top clot substance.
All 11 patients were transferred from a prophylactic dose of acetylsalicylic acid to apixaban 5 mg twice daily immediately before the research, in conformity with the rules for switching to another option of antithrombotic therapy.
Statistical results processing was executed using the IBM SPSS Statistics 22.0 program. To test the null hypothesis, the Mann-Whitney criterion was used to match the advised independent groups and was considered a statistically significant difference at the significance level of p ≤ 0.05. Indicates quantitative indicators are presented as Me [LQ; Uq], where Me is the median, LQ (Q25) is the lower quartile, UQ (Q75) is the upper quartile.
3. Main results
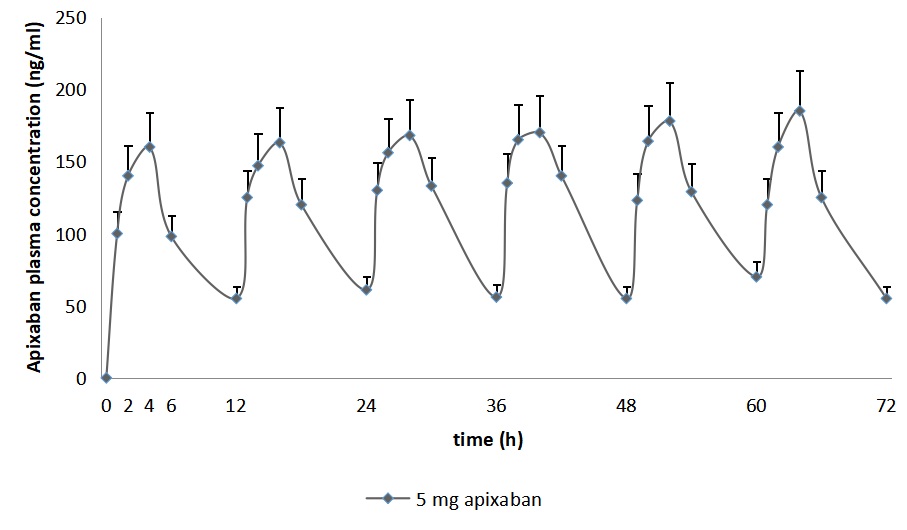
Figure 1 - Confidence intervals of 3-day monitoring of apixaban concentration at a dose of 5 mg twice daily in the blood plasma of patients with AFNE
When evaluating the pharmacodynamics of POAK by determining anti-Xa activity at pharmacokinetic analysis points during the first 12 hours, we resorted to researching its conjugation with the concentration of the studied anticoagulants. The results of the correlation analysis shown in Figure 2 indicate that there is a statistically substantial correlation between anti-Xa activity and plasma concentrations of the direct factor Xa inhibitor apixaban, begins to be determined only at ≈ 100-110 ng/ml of the drug.
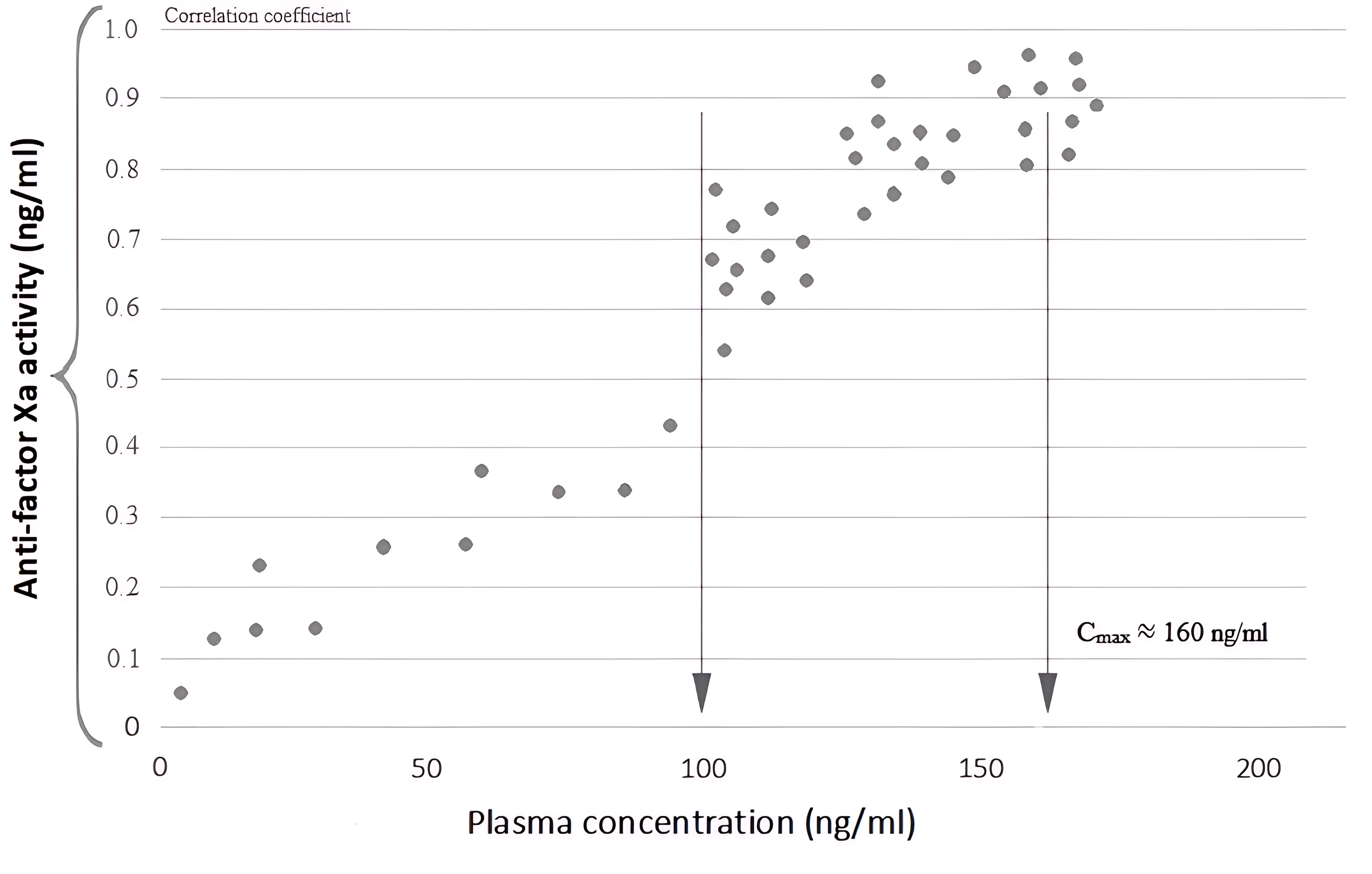
Figure 2 - Correlation between anti-Xa activity and plasma concentration of the direct factor Xa inhibitor, apixaban. The arrow indicates concentration levels corresponding to the geometric mean values of Cmax
Note: modified according to R. Kreutz et al., 2017
When analyzing the points of the LFPTEG and behavior of the hemostatic potential (HP) at the reference points of the study (Table 1), first of all, it is necessary to dwell on its initial characteristics. Indeed, previously conducted antiplatelet therapy provided moderate chronometric hypocoagulation at the initial stages of fibrinogenesis, assessed by the increase in t1 - 3.3 [2.8; 3.7] min. (p≤0.01) and t3 – 10.3 [8.0; 11.5] min. (p≤0.05), with a decrease in activity of thrombin at the initial stages of proteolysis, assessed by TAC – 15.0 [12.5; 22.2] o.u. (p≤0.05). Probably, the initial moderate structural hypercoagulation (MAinitial = 695 [670; 735] o.u.) also has a compensatory nature in response to antiplatelet therapy. In parallel with this, a slight reduction in the organization time of the fibrin-platelet structure of the clot was recorded – t5 to 38.8 [32.3; 43.0] min. The combination of the described changes in HP in patients with AFNE actually served as the basis for changing the antithrombotic drug strategy due to the insufficient effectiveness of antiplatelet agents.
Table 1 - The values of the measured characteristics of LFPTEG by observation “points” in patients with non-valvular atrial fibrillation after twice daily dose of 5 mg of apixaban
Indicators | reference | originally | 2 hours | 4 hours | 6 hours | 12 hours |
t1 (min.) | [0.4;1.0] | 3.3 [2.8;3.7]* | 6.6 [4.8;7.5]*# | 8.0 [5.0;9.0]*# | 5.1 [3.8;6.0]*# | 4.4 [3.5;5.3]*# |
ICC (r.u.) | [10;36] | 10.0 [6.8;16.1] | 3.2 [1.3;10.4] | 4.5 [2.8;10.0]*# | 3.5 [1.7;6.6]# | 7.1 [4.7;14.3] |
TAC (r.u.) | [25;45] | 15.0 [12.5;22.2]* | 9.6 [7.4;11.8]*# | 9.0 [6.0;12.0]*# | 11.8 [10.0;15.6]*# | 13.1 [10.5;16.7]*# |
t3 (min.) | [4;8] | 10.3 [8.0;11.5]* | 18.0 [14.5;21.7]*# | 18.0 [13.0;20.0]*# | 13.5 [10.2;15.5]*# | 11.4 [9.0;13.5]* |
ICD (r.u.) | [30;50] | 35.7 [32.6;43.1] | 23.5 [19.8;28.6]*# | 21.8 [20.1;26.9]*# | 20.8 [19.7;24.5]*# | 23.8 [22.2;27.8]*# |
t5 (min.) | [23.3;39] | 38.8 [32.3;43.0] | 40.8 [36.3;45.0] | 45.0 [36.5;49.5]*# | 44.0 [40.0;46.0]*# | 36.0 [32.0;42.0] |
МА (r.u.) | [400;650] | 695 [670;735]* | 577.5 [559;635]# | 590 [550;620]# | 533 [480;565]# | 533.0 [495;560]# |
Note: * - statistically significant differences from the reference values (p≤0.05);
# - statistically significant differences from the originally values (p≤0.05)
Plasma concentration within 2 hours after taking apixaban 140 [138; 160] ng/ml, according to LFPTEG, chronometric (t1-6.6 [4.8; 7.5] min. and t3 – 18.0 [14.5; 21.7] min.) (p≤0.05) and structural (TAC – 9.6 [7.4; 11.8] r.u. and ICD – 23.5 [19.8; 28.6] r.u.) decreased blood clotting is formed both in relation to the reference values and to the initial data (p≤0.05). The peak of the structural and time-series decrease in coagulability is statistically quite extended in t1-8.0 [5.0; 9.0] min., t3-18.0 [13.0; 20.0] min. and t5-45.0 [36.5; 49.5] min. and a significant reduction in TAC to 9.0 [6.0; 12.0] o.u., and ICD to 21.8 [20.1; 26.9] o.u. by the 4th hour of the study and the concentration of the drug, recorded within 160 [156; 179] ng/ml. At the 6th and 12th hours of monitoring, structural and chronometric hypocoagulation is preserved in the LFPTEG characteristics, confirmed by a significant decrease in TAC and MA in relation to the reference values and ADC in relation to the initial values (p≤0.05 for all cases). Chronometric hypocoagulation at these hours is preserved for t1 and t3 in relation to their reference values.
4. Conclusion
First of all, it should be noted that the change of therapy with anticoagulant together antiplatelet agents to a direct factor Xa inhibitor – apixaban at a dosage of 5 mg 2 times a day did not negatively affect the condition of the subjects.
Observe effectiveness of apixaban in antithrombotic prophylaxis/therapy in research group of peoples with AFNE by determining the activity of the anti-Xa factor is acceptable at plasma concentrations of this NOAC from 100-110 ng/ml or more. Evaluation of apixaban pharmacokinetics in twice-daily dosing showed that drug concentrations of apixaban were only measured from the first to the sixth hour after POAK administration during 72-hour monitoring.
Thus, in almost half of the supervision period of peoples' group of AFNE who are on anticoagulant therapy, data on the clinical efficacy of POAC using the anti-Xa factor activity test is absent. In that regard, the most adequate method for monitoring the pharmacodynamics of anticoagulant medicament with the specified discreteness of use is the LFPTEG method, more sensitivity of that plasma concentrations of the POAK inside of 45 ng/ml are sufficient.
