RESEARCH OF TREATMENT PROCESS OF WELL WATER WITH A HIGH CONCENTRATION OF MANGANESE AND IRON
Юдаков А.А.1, Чириков А.Ю. 2, Перфильев А.В. 3, Бадулин Ю.М. 4,
Слесаренко В.В. 5, Червонецкий Д.В. 6
1Доктор технических наук, профессор, Федеральное государственное бюджетное учреждение науки Институт химии Дальневосточного отделения Российской Академии наук;
2Ведущий инженер-технолог, Федеральное государственное бюджетное учреждение науки Институт химии Дальневосточного отделения Российской Академии наук;
3Кандидат химических наук, Федеральное государственное бюджетное учреждение науки Институт химии Дальневосточного отделения Российской Академии наук;
4Ведущий инженер-технолог, Федеральное государственное бюджетное учреждение науки Институт химии Дальневосточного отделения Российской Академии наук;
5Доктор технических наук, профессор, Федеральное государственное бюджетное учреждение науки Институт химии Дальневосточного отделения Российской Академии наук;
6Научный сотрудник, Федеральное государственное бюджетное учреждение науки Институт химии Дальневосточного отделения Российской Академии наук.
ИССЛЕДОВАНИЕ ПРОЦЕССА ОЧИСТКИ СКВАЖИННОЙ ВОДЫ С ВЫСОКИМ СОДЕРЖАНИЕМ МАРГАНЦА И ЖЕЛЕЗА
Аннотация
В статье приведены результаты экспериментальных исследований по деманганации скважинных вод, в которых концентрация марганца превышает 5 мг/л, железа – 20 мг/л. Приведено описание разработанной технологии деманганации. Представлены результаты испытаний на станции водоподготовки г. Комсомольск-на-Амуре.
Ключевые слова: деманганация, железо, марганец, очистка воды.
Yudakov A.A.1, Chirikov A.Yu. 2, Perfilev A.V. 3, Badulin Yu.M. 4,
Slesarenko V.V. 5, Chervonetskiy D.V. 6
1Doctor of Technical sciences, professor, Institute of Chemistry, Far-Eastern Branch of Russian Academy of Sciences;
2Lead Process Engineer, Institute of Chemistry, Far-Eastern Branch of Russian Academy of Sciences;
3PhD in Chemistry, Institute of Chemistry, Far-Eastern Branch of Russian Academy of Sciences;
4Lead Process Engineer, Institute of Chemistry, Far-Eastern Branch of Russian Academy of Sciences;
5Doctor of Technical sciences, professor, Institute of Chemistry, Far-Eastern Branch of Russian Academy of Sciences;
6Research Fellow, Institute of Chemistry, Far-Eastern Branch of Russian Academy of Sciences
RESEARCH OF TREATMENT PROCESS OF WELL WATER WITH A HIGH CONCENTRATION OF MANGANESE AND IRON
Abstract
In article we present the experimental results on demanganation of well water where the manganese concentration exceeds 5 mg/l and iron concentration – 20 mg/l. The developed technology for demanganation is described. The results of experiments at water treatment plant of Komsomolsk-on-Amur are presented.
Keywords: iron, manganese, manganese removal, water purification.
Introduction
Manganese is contained in Earth crust abundantly and typically occurs together with iron. If manganese concentration exceeds 0.1 mg/l water becomes unfit for drinking, industrial and service purposes, making spots appear on sanitaryware and leaving unwanted water taste. On internal walls of pipelines some sediments appear and go off as brownish black membrane. In most cases, iron compounds are great part of that sediment, besides manganese.
Bivalent manganese contained in underground waters is very slowly oxidizes to trivalent and tetravalent form with oxygen dissolved in water or with other oxidizers.
As it was shown by chemical analyses manganese concentration in water from artesian wells of Komsomolsk-on-Amur may reach 2.5~7.4 mg/l i.e. significantly exceeds common values. Today dissolved manganese concentration in water treated at Komsomolsk-on-Amur water treatment plant may reach 0.7~0.9 mg/l which exceeds SanPiN 2.1.4.1074-01 [1] norms 7~9 times. Besides, black suspended matter is contained in water – particles of oxidized manganese membrane developed in the foiling of high-rate filters (pic. 1).
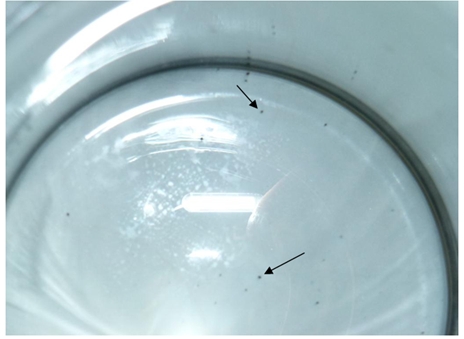
Pic. 1 – Oxidized manganese membrane particles in service water
The purpose of this research was to develop a technology for demanganation of well water delivered to Komsomolsk-on-Amur network ensuring water quality parameters as per SanPiN 2.1.4.1074-01 and providing good quality drinking water for end-users.
Materials and methods
Technology consists of stages of gradual adding in water under treatment an alkaline agent (NaOH), an oxidizer (KMnO4) and a coagulant (aluminum polyoxichloride) followed by filtration of the sediment developed on catalyst filing (MFO-47) and fine post-treatment via granular filling layer (Filter-AG). Concentrations of chemicals were as follows: NaOH solution – 20 g/l, KMnO4 solution – 5 g/l, aluminum polyoxichloride solution – 3 g/l.
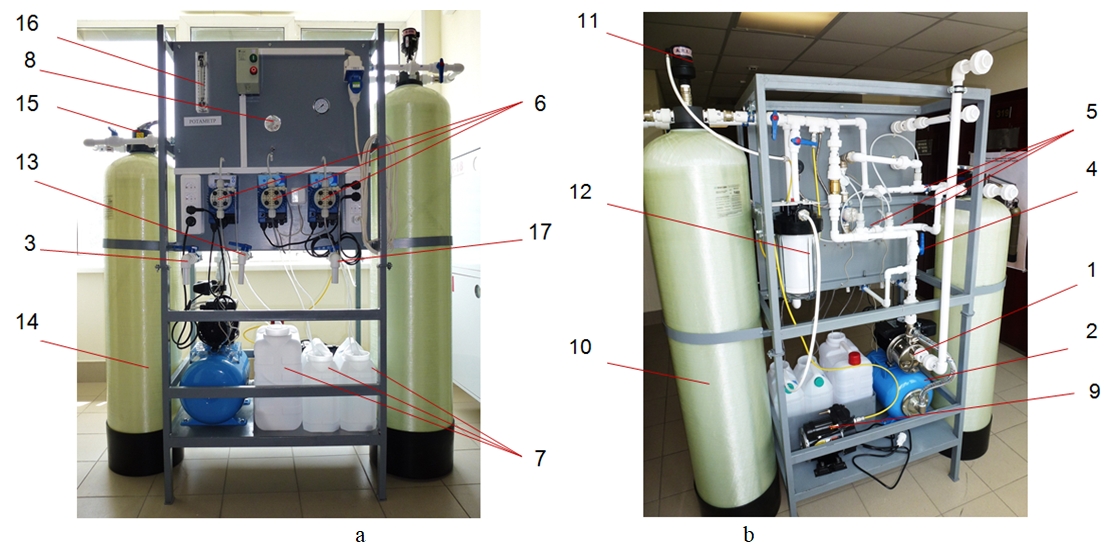
To study demanganation of well water, small-scale experimental plant was designed and made (WTP – water treatment plant) (pic. 2).
The plant is a mobile station for water treatment ensuring treatment at least as per SanPiN 2.1.4.1074-01. The water treatment technology utilized ensures water treatment from coarsely dispersed, colloid and dissolved foreign matters. WTP may treat water from artesian wells and surface water sources used for technological and drinking purposes. The plant is not for treatment of sewage water containing high level of color and silt.
Pic. 2 – General view of experimental plant:
a – control panel view; b – machinery view
(1 – feed pump; 2 – hydraulic accumulator; 3 – source water intake; 4 – control valve; 5 – nozzles; 6 – dispensing pump; 7 – chemicals solutions tanks; 8 – flow meter; 9 – compressor; 10 – air-stripping tower; 11 – ventilation valve; 12 – residual ozone burning filter; 13 – water sampling after air-stripping tower; 14 – filter; 15 – manual tip; 16 – rate-of-flow meter; 17 – water sampling after filtration)
Table 1 represents the specifications and dimensions of WTP.
Table 1 – Specifications of WTP
| Specification | Value |
| Plant’s capacity, m3/hr | 2.1 |
| Hydraulic system pressure, MPa (pressure relay installed) | 0.2 – 0.3 |
| Compressor capacity, m3/hr | 2.5 |
| Compressor discharge pressure, MPa | 0.7 |
| Maximal use of chemicals after metering devices, l/hr | up to 2.4 |
| Plant’s weight without water filled, kg | ≈175 |
| Plant’s weight with water filled, kg | ≈350 |
| Plant’s dimensions (without filters), m | 0.6×0.85×1.81 |
| Voltage, V | 220 |
| Power consumption, kW | 1.5 |
WTP operation principle is based on the following water treatment technologies:
- correction of primary water (alkalization, acidification, etc.) to change the media parameters to optimize water treatment process and improve final parameters of water treated by feeding chemicals via dispensing pumps;
- oxidizing admixtures dissolved in primary water by gaseous or liquid oxidizers to turn them into colloid form, done by mixing water with air, ozone, chlorine, potassium permanganate or other oxidizer selected for that purpose;
- direct-flow coagulation based on primary treatment of water with chemicals (coagulants and flocculants) by feeding required volume of chemicals into water under treatment via dispensing pumps;
- water filtration via pressurized clarifying mechanical wave filters with special filling;
- water treatment from hard-to-remove admixtures using extra sorption filters.
Water for treatment in the experimental plant was taken at three main technological stages of water treatment station: from cyclonic aerator, from high-rate filter surface, service water pipeline.
Capacity of the experimental plant was varying from 5 to 25 l/min. If the plant showed maximal permissible result for manganese at the preceding capacity, the next capacity was not set.
Manganese and iron concentrations were determined by atomic absorption analysis method at SHIMADZU AA-6200 plant.
Results and discussion
Study of water demanganation modes on experimental WTP
Mode #1. Treatment of water from cyclonic aerator
Here, water from cyclonic aerator means the water taken from wells and undergoing the first stage of treatment – aeration in cyclonic aerator of Komsomolsk-on-Amur water treatment station.
From pipeline to intake, water was fed to the experimental plant where alkaline solution was added to increase pH and shifting redox potential towards oxidation, KMnO4 as oxidizer and aluminum polyoxichloride as coagulant. Water treated with chemicals was fed in air-stripping tower being the reactions chamber where air agitation was intensively applied and upon 15 minutes’ stay was fed to filtration columns. In filtration columns water was sequentially filtered via MFO-47 filling and then via Filter-AG.
Specifics of the experimental WTP’s operation in mode 1 were in high dirt load for the plant. High content of iron and manganese caused increased consumption of chemicals causing, together with coagulant, great volume of sediment. That sediment is clogging the first stage filter (MFO-47) and then the second stage filter (Filter-AG).
Existing technological scheme of treatment facilities water upon aeration and ozone adding is delivered to horizontal tanks. Thus, high rate filters experience less load in connection with iron hydroxide – filters are just decreasing residual content of iron in water. In the experimental plant, all the mass of oxidized and hydrolyzed iron is delivered to high rate pressurized filtration, filter operating with high dirt load. The situation is enhanced by adding great doses of alkaline agent to achieve high pH, while charging stabilization of iron hydroxide microsols and/or early hydrolysis of coagulant salts with no impact on suspended matter.
Extra addition of oxidizer solutions (KMnO4) makes that system more difficult to find a way to reduce manganese content in water.
Mode #2. Water treatment after clarifiers
Here water after clarifiers means the water which passed treatment in aerator (ozonation) in the technological chain of water treatment station and is delivered to upper parts of high rate filters for final treatment. Water after clarifiers is free from hydrogen sulfide, carbon dioxide, almost all dissolved iron and partly manganese.
Water treatment mode is similar to mode 1.
Drinking water may be received from process water directly in places and plants identical to the experimental plant.
Mode #3. Treatment of network water
Here network water means the water delivered to end-users’ network.
Three modes of network water treatment were studied: two-stage filtration via filling layer (MFO-47, Filter-AG) (mode 3a); coagulation with further two-stage filtration (mode 3b); full treatment with chemicals and further two-stage filtration (mode 3c, similar to mode 1).
Table 2 lists the results of network water treatment in various modes.
Table 2 – Results of network water treatment
| Mode | Water consumption, l/min. | Initial manganese concentration, mg/l | Final manganese concentration, mg/l |
| 3a | 5 | 0.186 | 0.068 |
| 10 | 0.066 | ||
| 15 | 0.048 | ||
| 3b | 5 | 0.186 | 0.091 |
| 10 | 0.083 | ||
| 15 | 0.089 | ||
| 3v | 5 | 0.186 | 0.014 |
| 10 | 0.014 |
As seen from Table 2, manganese concentration in any mode of network water filtration drops below the level set forth by SanPiN 2.1.4.1074-01.
Higher manganese concentrations (3b mode) are explained by small acid reaction of coagulant which decreases water’s pH and the effectiveness of catalyst filling MFO-47. That mode, despite the manganese parameters being below SanPiN norms is unreasonable due to non-rational use of coagulant.
The specifications of network water obtained and its high organoleptic features are proving conclusively that one of the ways to deliver high quality drinking water to end-users is to install local small-scale water treatment plants of required capacity similar to the experimental WTP.
Demanganation experiments results are represented in Table 3.
Table 3 – Demanganation experiments results
| Mode | Water type | Water consumption, l/min. | рН of primary water | Temperature of primary water, °С | Initial manganese concen-tration, mg/l | Final manganese concen-tration, mg/l | Initial iron concen-tration, mg/l | Final iron concen-tration, mg/l |
| 1 | Water from cyclonic aerator | 5 | 6.36 | 8.1 | 5.120 | 0.774 | 20,050 | Not found |
| 2 | Water after clarifiers | 15 | 6.54 | 8.8 | 4.284 | 0.020 | 0,564 | Not found |
| 3 | Network water | 10 | 6.54 | 8.8 | 0.186 | 0.014 | 0,032 | Not found |
Conclusion
Positive results obtained in the course of the works showed that the technology developed may be applied for treatment of drinking and process/service industrial water. It is most effective at stations of drinking water treatment and final treatment installed at food processors, small enterprises making products utilizing highly treated water, small service enterprises for delivering drinking water to end-users, at any enterprise/organization of any other sector where increased requirements are brought to the quality of water used.
Acknowledgements
This work was financially supported by the Ministry of Education and Science of the Russian Federation, by order of P 218, the contract № 02.G25.31.0035-225 dated 12 February, 2013 between Open Joint Stock Company "Far East Plant "Zvezda" and the Ministry of Education and Science of the Russian Federation.
References
SanPiN 2.1.4.1074-01 norms Drinking water. Hygienic requirements to quality of water of centralized drinking water supply systems. – Enact 01.01.2002. – 53 p.