ЭЛЕКТРОПЛАЗМЕННАЯ ПЕРЕРАБОТКА ЗОЛООТХОДОВ ОТ СЖИГАНИЯ В СТЕКЛОКРИСТАЛЛИЧЕСКИЕ ЛИТЫЕ МАТЕРИАЛЫ
ЭЛЕКТРОПЛАЗМЕННАЯ ПЕРЕРАБОТКА ЗОЛООТХОДОВ ОТ СЖИГАНИЯ В СТЕКЛОКРИСТАЛЛИЧЕСКИЕ ЛИТЫЕ МАТЕРИАЛЫ
Аннотация
В статье рассмотрены характеристики золоотходов, полученных в мусоросжигательных печах (Китай), с целью установления возможности их использования в качестве сырья для производства стеклокристаллического литья электроплазменным методом. Изучение гранулометрического, минералогического и химического состава сырья проводилось с помощью дисперсионно-гравиметрического, микроскопического, спектрального и рентгенофазового анализов. В ходе исследования установлены истинная плотность, удельная поверхность, гранулометрический, химический и минералогический составы сырья и получаемых из него каменных отливок с расчетом модуля кислотности, который составил Мк = 2,73, что позволяет производить отливки из однокомпонентной шихты (золоотходов). В процессе электроплазменной плавки сырья была получена кальций-силикатная каменная отливка, представляющая собой закристаллизованный сплав заданной геометрической формы из кварца (SiO2) в стеклофазе. Полученные в ходе проведенных исследований данные позволяют рекомендовать электроплазменное плавление как способ переработки золоотходов от сжигания в расплав с низкими энергозатратами (0,8–1,0 кВт-ч/кг), а полученный продукт классифицировать как кальций-силикатное стеклокристаллическое литье.
1. Introduction
In recent years, special attention has been paid to the efficient use of man-made, recycled and recycled waste in the production of various building materials and products
. The resource consumption of modern society generates a large amount of substandard waste stored and accumulated in landfills and landfills, which worsen the environmental situation near their locations . One of the most reliable and economically feasible ways to eliminate waste worldwide is its sorting and incineration at incinerators and plants called recycling . But when burning any type of garbage (municipal, industrial, etc.), a solid, non-flammable residue inevitably forms. Therefore, industrial incineration processing leads to the accumulation of huge amounts of ash waste from incineration in the form of powdered dust . In addition to being highly dispersed and prone to dusting, the waste presented is low-resistant, leachable in aggressive agents (mineral and organic acids), highly hygroscopic, susceptible to chemical weathering, and therefore substances of very limited use. But despite this, ash waste from incineration is a valuable secondary resource because ash dumps in landfills, with thoughtful and rational processing, can serve as sources of raw materials for other industries . To achieve maximum efficiency (minimizing costs and environmental risk), it is necessary to implement integrated technological solutions in which the use of some waste leads to the neutralization and effective processing of others .One of the ways to process ash from incineration may be to melt them in order to obtain a melt with further production of stone-like cast materials based on it. Preliminary experiments on electroplasmic melting of ash waste have shown the fundamental possibility of obtaining a melt and producing stone casting from it
. The study of the obtained products showed that its composition is represented by a quartz-containing SiO2 phase with a concentration of 25,32%. The X-ray image also shows a solid halo of a glass phase containing this phase. Therefore, during further research, the following tasks were set: to calculate the actual density, specific surface area, granulometric, chemical and mineralogical compositions of raw materials to determine the content of calcium and silicon oxides, which determine the formation of the required structure during melting and shaping; to calculate the modulus of acidity of the melt to solve the problem of priming during melting; to clean the exhaust gases, formed during the melting of ash waste containing volatile compounds; in an electroplasma installation designed according to the type of electromagnetic reactor, a melt is obtained from which, by pouring into side molds, a stone-like casting of specified geometric shapes is developed.The search for new raw materials, as well as ways of processing them to obtain glass-crystalline materials and products, is an urgent task for the needs of the construction industry
. In addition, significant characteristics of the physical and technological properties of these materials such as high abrasive, chemical and thermal resistance, long service life have predetermined their use as cladding and cast products resistant to abrasive wear. Thus, glass-crystal casting tiles are used as lining linings for flues, bunkers, cleaners, as well as floors and gutters of various equipment that can withstand a large number of freeze-thaw cycles . However, a limiting factor in the synthesis of these materials is high gas formation and the presence of potentially harmful substances in the ash, which complicates the production processes . Based on these facts, the purpose of this study was formed — to study the most important characteristics of substandard raw materials in the form of ash waste from incineration and to establish the possibility of its use in the production of cast glass crystal products (stony materials) by melting in an atmospheric environment using the energy of arc electroplasm with associated purification of the resulting flue gases. In comparison with the currently existing methods of synthesizing the presented materials, this method is easier to implement, and therefore preferable from the point of view of the possibility of organizing further production , .2. Materials and methods of research
The object of the research was fine-grained ash formed as a residue during the incineration of municipal waste at incinerators in China. The following scientific equipment was used in the work, provided by the PROGRESS Central Research Institute of ESSUTM, as well as the BSC SB RAS: Le Chatelier flask (Russia); electronic scales Sigma-scale 100/0,001 (USA); device for determining the specific surface area of powders PSX-2 (Russia); mechanical dispersion device (sieve analyzer) A-20 (Russia); digital polarizing microscope Altami POLAR 1 (Russia); X-ray diffractometer D8 Advance AXS BRUKER (Germany); atomic absorption spectrometer Solaar M6 Thermo Electron (USA); synchronous thermal analysis device STA 449 F1 Jupiter (Germany); capillary electrophoresis unit Drops-105 (Russia). The chemical and mineralogical compositions of raw materials and products were studied using microscopic, spectral and X-ray phase analyses. The fractional composition was studied using a sieve and gravimetric method, and the specific surface area was calculated using a PSX-2 device.
Optical microscopy in transmitted polarized light was used to determine the morphological characteristics of the raw material and the particle size, which determines the structure and dispersion of the material (Fig. 1).
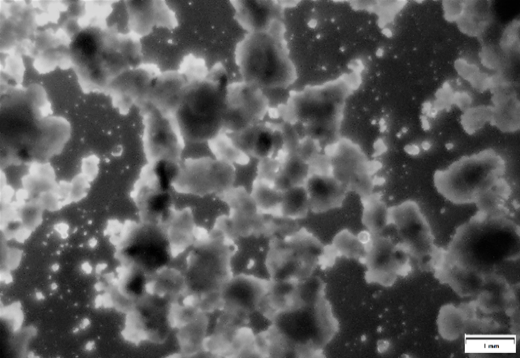
Figure 1 - Powdered ash of incineration
Note: 40x
Further studies were carried out to establish the true density, fractional composition and specific surface area of the raw material
. The density was calculated by the volume-weight method using a Le-Chatelier flask using formula 1:where m — is the mass of waste consumed in the experiment; v — is the volume of liquid after pouring the raw materials.
The specific surface area of powdered ash was determined on a PSX-2 device using the formula 2:
where K — is the constant of the device for a pair of plates between which a column of liquid was observed to fall during a time of τ, sec.; M – is the value determined from the measured values of the layer height H and air temperature (the values of K and M are contained in the device passport); m – is the weight of the suspension, g.
The grain composition was determined using a mechanical dispersion device (A-20 sieve analyzer) with a standard set of sieves. The data on the ash waste study are presented in Table 1.
Table 1 - Granulometric composition and specific surface area of ash waste
Fraction, mm | 0,3 | 0,2 | 0,1 | 0,05 | <0,05 |
Content, % | 6,24 | 18,20 | 55,60 | 18,24 | 1,72 |
Specific surface area, cm2/g | 645 | 1027 | 1572 | 2064 | 3704 |
The calculated true density of ash waste was ρ = 2707 kg/m3, and the fractional composition and specific surface area indicated qualitative changes and transformations that occurred with the original substance (sorting, crushing and separation followed by thermochemical action).
The possibilities of processing any materials directly depend on knowledge of their chemical composition, which determines the methods of exposure to them. Therefore, further studies conducted by methods of physico-chemical analysis were aimed at establishing the chemical composition and determining the main components of ash waste from incineration.
To study the chemical and mineralogical composition of raw materials according to GOST ISO 5725-6-2003, an analytical sample of 0,1 mm fraction was obtained by averaging and quartering (it is the dominant and therefore the host fraction). The chemical analysis of the ash was carried out using titrimetric, gravimetric, and photometric methods, as well as a Solaar M6 Thermo Electron atomic absorption spectrometer. The chemical composition of the ash waste is presented in Table 2.
Table 2 - Chemical composition of ash waste from incineration
Substance | SiO2 | Al2O3 | FeO+Fe2O3 | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | SO3 | CuO | ZnO | C | Cl |
Mass fraction, % | 12,84 | 4,82 | 2,63 | 2,78 | 26,43 | 3,10 | 2,85 | 0,51 | 2,60 | 5,28 | 0,74 | 1,29 | 24,43 | 9,70 |
The chemical composition of the studied raw materials allowed us to establish a high concentration of carbon, calcium, silicon, potassium and sodium contained in it, as well as phosphorus and chlorine, which form the basis of most organic and inorganic substances and compounds that form waste. Thus, it was shown that ash waste consists of almost 25% of the carbon component of the "organic part", while the remaining 75% is represented by a complex composition of elements forming an "inorganic complex" or mineral mass. Qualitatively and quantitatively, the ash composition is most likely formed not only from the elements included in the waste, but also introduced, passing into the substance from burning and supporting gorenje energy carriers (coal, fuel oil, etc.)
, .To identify the crystallographic phases that make up the incineration ash, its X-ray phase analysis (XFA) was performed
. X-ray diffraction was performed on a BRUKER AXS D8 Advance X-ray diffractometer in Cu Kα radiation. X-ray images were taken in the range of angles of 2 θ from 0° to 70° in increments of 0,03°, with a scanning speed of 2.0 degrees/min. The data bank of PDF-2 powder radiographs of organic, inorganic, mineral and synthetic compounds was used for the analysis. The quantitative phase ratio was determined in the TOPAS 4.2 program by the Rietveld method.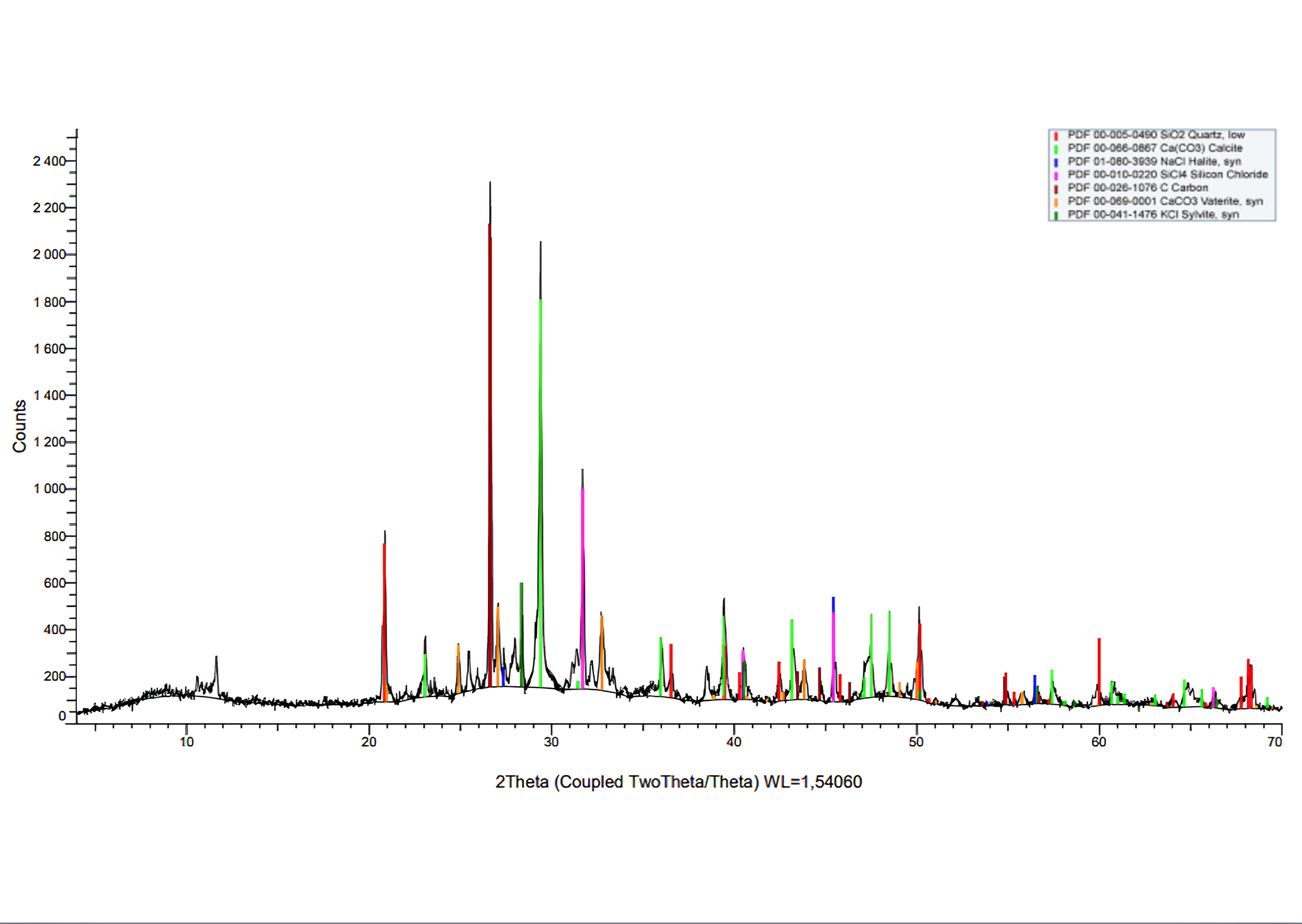
Figure 2 - X-ray phase diagram of ash waste from incineration
Note: the phases of quartz, calcite, halite, sylvinite, and carbon are presented
The presence of elements such as carbon, chlorine, sulfur and phosphorus in the composition of ash potentially complicates the process of its processing. Therefore, additional research was required to establish the thermal stability of the raw materials
. The tests were carried out on a synchronous thermal analysis device STA 449 F1 Jupiter.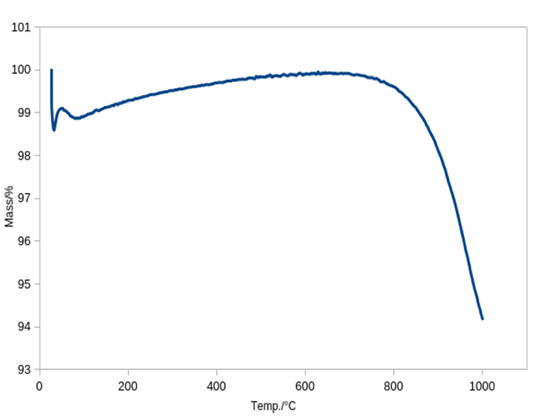
Figure 3 - TGA diagram of phase transformations of ash waste
3. The results of the study and their discussion
As a result of the study of the composition and structure of the raw material, reliable data were obtained on its melting to obtain a silicate melt and the production of glass-crystal casting from it
, . For this purpose, a series of experiments was conducted, with the development of smelting modes using electroplasma melting equipment designed in the form of an electromagnetic process reactor. Thermal energy in the electric arc zone of the reactor chamber was generated by plasma-ohmic heating. Thus, this zone was both a zone of heat energy generation and a zone of its absorption, making it possible to regulate (by increasing or decreasing the current strength) the melting time of raw materials in it , , . Structurally, the operating principle of the reactor is presented as follows: an electric arc burns in the reactor chamber between the three-phase electrodes, and electromagnetic windings serially connected to the electrodes create a rotating magnetic field that simultaneously mixes the melt, thereby contributing to its homogenization and eliminating non-melting zones. The schematic diagram of the plant for electroplasma melting of raw materials is shown in Figure 4.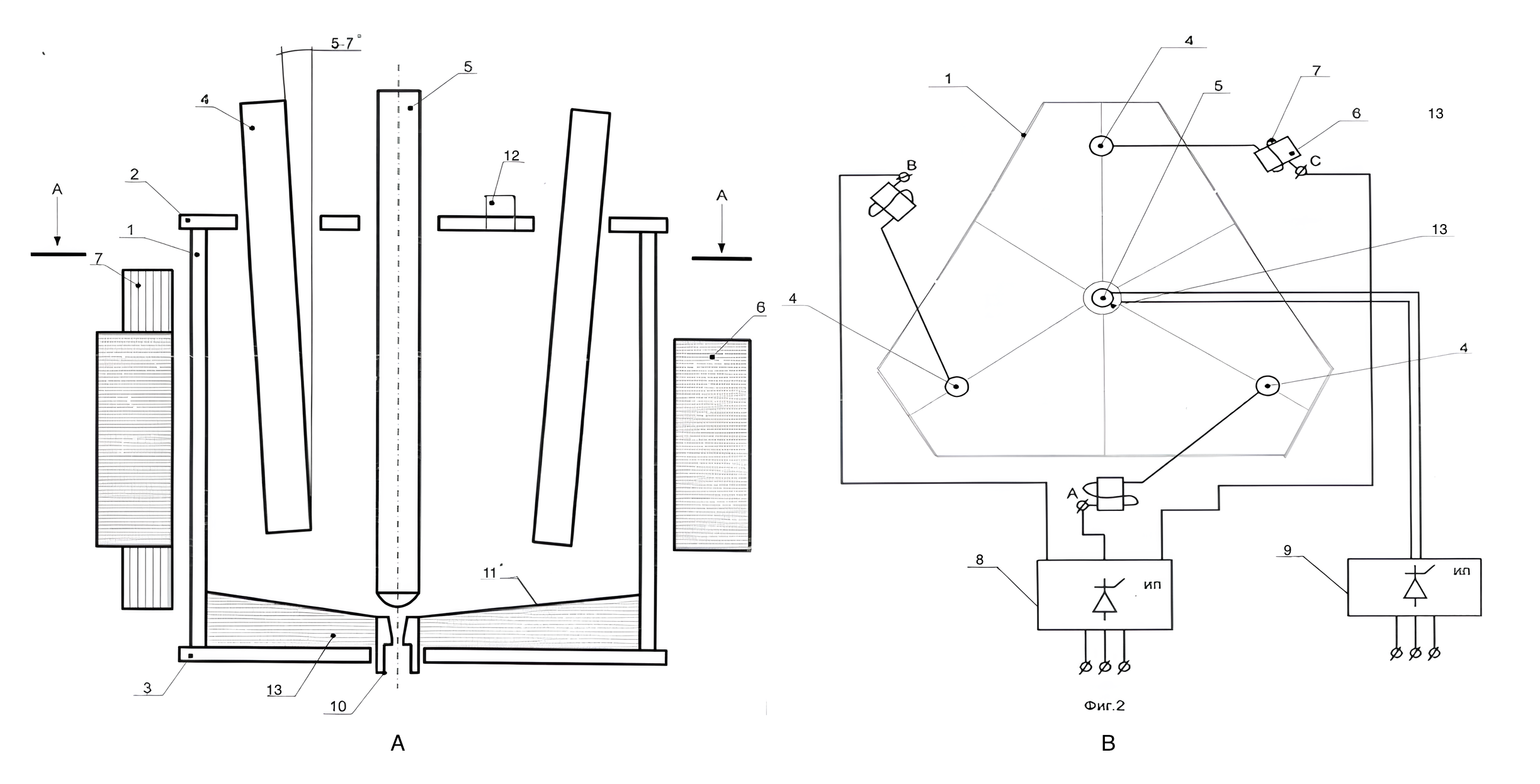
Figure 4 - Longitudinal (A) and transverse (B) sections of an electromagnetic process reactor
Note: 1 – reaction chamber; 2 – water–cooled lid; 3 – water–cooled bottom; 4 – rod electrodes (3 pcs.); 5 – rod locking electrode; 6 – pole tip; 7 – series winding; 8 – power source; 9 – additional power source for heating the jet; 10 – device for removing the melt (letka); 11 – lined bottom of the chamber; 12 – pipe into the reaction chamber for feeding raw materials; 13 – lining
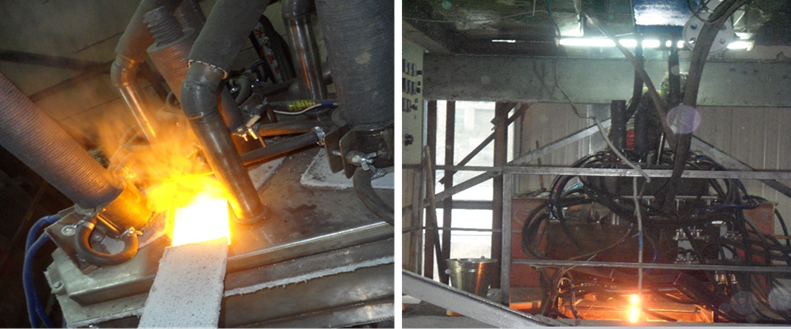
Figure 5 - Electroplasmic melting of raw materials, as well as the moment of fusion of the melt with the production of stone casting

Figure 6 - Discharge of the melt jet from the reactor vessel and its casting into molds

Figure 7 - Examples of cast materials (tiles and cylinders in section) obtained from the melt

Figure 8 - Water-bubbling gas purification system, formed during the melting of ash waste from incineration
Table 3 - Cationic and anionic composition of scavenger water
№ | Cations | Conc., mg/l | Anions | Conc., mg/l |
1 | NH4 | 5,45 | Chloride ions | 401 |
2 | K | 248 | Nitrite ions | 6,91 |
3 | Na | 490 | Sulfate ions | 90,8 |
4 | Li | 0,210 | Nitrate ions | 2,84 |
5 | Mg | 51,9 | Fluoride ions | 8,93 |
6 | Ca | 79,6 | Phosphate ions | 10,9 |
7 | – | – | Bromide ions | 9,08 |
8 | – | – | Iodide ions | 3,76 |
Further studies of the resulting casting were aimed at determining its phase-mineralogical composition based on X-ray phase analysis data, as well as studying the main components of the chemical composition by methods of physico-chemical analysis with the calculation of the modulus of acidity.
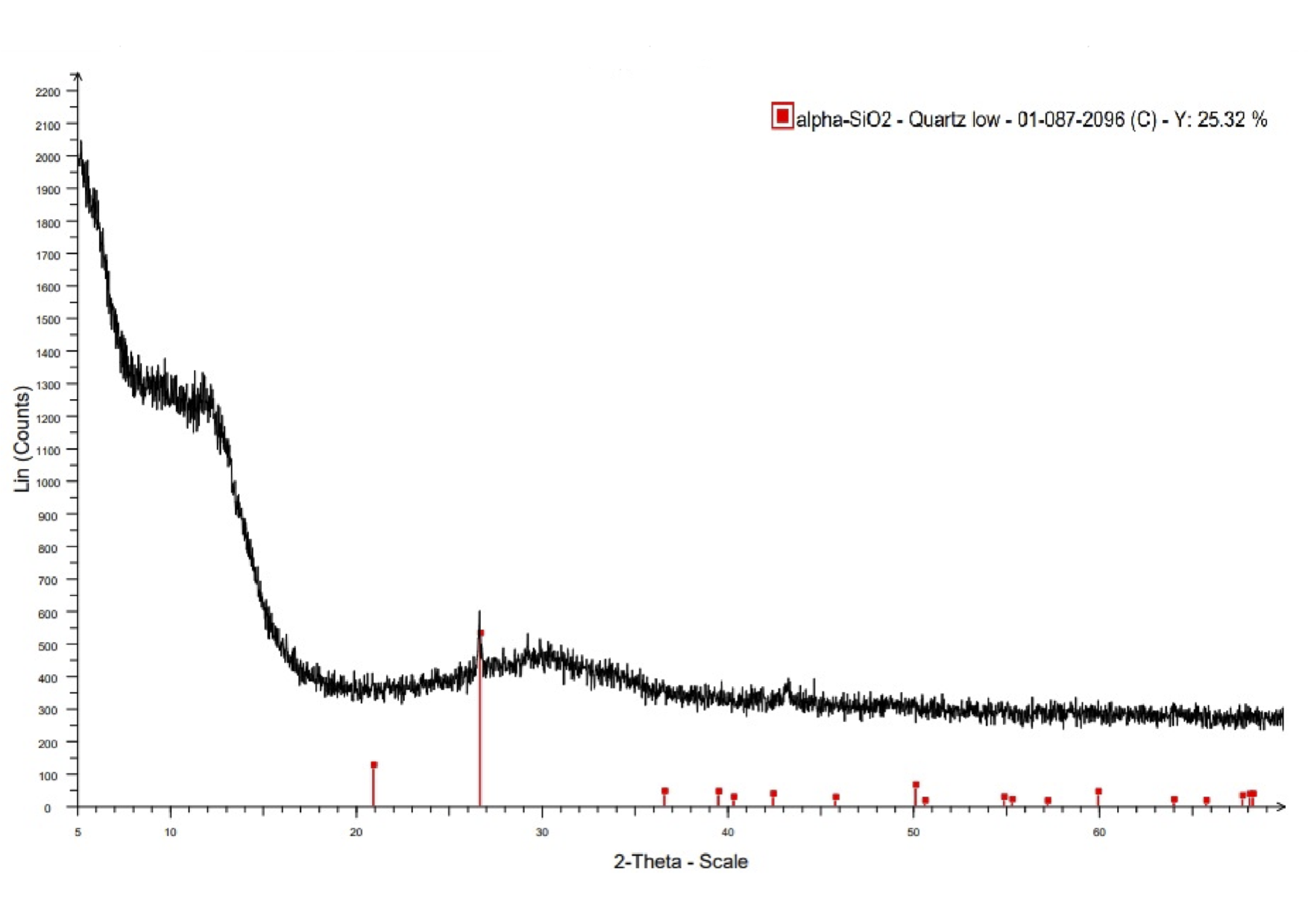
Figure 9 - X-ray diffraction pattern of glass crystal casting obtained by electroplasma method
The final stage of the research was the determination of the main components of the chemical composition of the casting produced from the melt by methods of physico-chemical analysis with the calculation of its modulus of acidity. The quantitative data of the casting obtained using thermal plasma are presented in Table 4.
Table 4 - Chemical composition of glass crystal casting obtained from waste incineration ash
Substance | SiO2 | Al2O3 | FeO + Fe2O3 | MgO | CaO | Na2O | K2O | TiO2 | P2O5 |
Mass fraction, % | 53,41 | 14,76 | 1,84 | 6,64 | 18,32 | 2,49 | 0,45 | 0,62 | 1,47 |
Then, after determining the chemical composition, the acidity modulus was calculated, characterizing the acid-base properties
. This module is used for a preliminary assessment in order to determine the suitability for producing a melt and casting from it and is determined by the formula 3:where SiO2, Al2O3, CaO, MgO are the content of basic oxides, mass %.
To obtain a melt that contributes to the production of high-quality casting, the modulus of acidity of the rock must be in the range of values from two to six
. In this case, Mk = 2.73, which makes it possible to obtain glass crystal casting in the process of electroplasma melting from single-component raw materials without mixing.The data obtained in the course of the conducted studies allow us to recommend electroplasma melting as a method of processing ash waste from incineration into a melt with low energy consumption (0,8-1,0 kWh/kg), and classify the produced product as a calcium-silicate glass-crystal casting
.4. Conclusion
In the course of the study, the following results were obtained:
1. The actual density, specific surface area, granulometric, chemical and mineralogical (X-ray phase analysis) compositions of raw materials consisting of ash waste from incineration have been calculated. An increased content of calcium and silicon oxides has been established, which determine both the formation of the glass crystal structure of the casting and the stability of the melting and shaping process. The high content of calcium oxides also indicated that the composition of the resulting casting would be calcium silicate.
2. The modulus of the melt acidity was calculated, which amounted to Mk = 2,73, which made it possible to obtain stone casting in the process of electroplasma melting from single-component raw materials without its mixing.
3. With the help of a simple water-bubbling system, the exhaust gases generated during the melting of ash waste were cleaned.
4. In an electroplasma installation designed according to the type of electromagnetic reactor, a melt was obtained, from which a glass crystal casting of specified geometric shapes was developed by pouring into side molds.
Thus, the conducted complex of studies allows us to recommend electroplasma melting as a method of processing ash waste from incineration into a melt with low energy consumption (0,8-1,0 kWh/kg), and classify the produced product as a calcium-silicate glass-crystal casting.
