НОВЫЕ УГЛЕРОДНЫЕ НАНО-АДСОРБЕНТЫ ИЗ СКОРЛУПЫ ОРЕХОВ МАКАДАМИИ В МЬЯНМЕ, ИСПОЛЬЗУЕМЫЕ ДЛЯ УДАЛЕНИЯ Н-БУТАНОЛА ИЗ ПАРОВОЗДУШНОЙ СМЕСИ
НОВЫЕ УГЛЕРОДНЫЕ НАНО-АДСОРБЕНТЫ ИЗ СКОРЛУПЫ ОРЕХОВ МАКАДАМИИ В МЬЯНМЕ, ИСПОЛЬЗУЕМЫЕ ДЛЯ УДАЛЕНИЯ Н-БУТАНОЛА ИЗ ПАРОВОЗДУШНОЙ СМЕСИ
Аннотация
Растительные отходы многочисленных производств Мьянмы в целом используют с низкой эффективностью. Наряду с этим данные научно-технической информации свидетельствуют, что на базе сходных по природе отходов можно получать продукты повышенной стоимости достаточно высокого качества, в частности, углеродные адсорбенты, ориентированные в основном на решение задач очистки производственных стоков и выбросов. Активные угли предлагают широкий спектр пористых структур и его поверхностной химии для адсорбции газов, которые используются для разработки практических процессов адсорбции с колебанием давления и температур для разделения и очистки газовых смесей. Активные угли часто предпочитают цеолитным адсорбентам в процессе разделения газов из-за их относительно умеренной силы адсорбции газов, что облегчает процесс десорбции. Рассмотрены три коммерческих применения активных углей:
а) удаление следов примесей из загрязнённого газа;
б) производство водорода из отходящего газа парового риформера метана;
в) производство азота из воздуха.
Также описаны четыре новых применения активных углей для разделения и очистки газов. Они включают в себя:
а) разделение смесей водорода и углеводородов путём селективного поверхностного потока более крупных молекул углеводородов через нано-пористую углеродную мембрану, полученную путём карбонизации полимерной матрицы;
б) осушку газа путём адсорбции при переменном давлении с использованием селективного для воды микропористого углеродного адсорбента, полученного путём поверхностного окисления гидрофобного углерода;
в) удаление путём селективной адсорбции и окисления на месте следов летучих органических соединений из воздуха с использованием композита углеродного адсорбента-катализатора;
г) хранение сжатого природного газа на углях с большой площадью поверхности.
В работе описана процедура приготовления, охарактеризованы показатели выхода и структурно-адсорбционных свойств активного угля паровой активации на базе скорлупы орехов макадамии, сопоставленные с таковыми на основе скорлупы орехов кокосовой пальмы и косточек сливы. Оценены кинетика и равновесие поглощения ими паров н-бутанола из их смесей с воздухом, проведено моделирование зависимостей по известным уравнениям. Сделан вывод о перспективности изученного пути переработки отходов орехов макадамии.
1. Introduction
More and more attention is being paid to the issues of engineering protection of the environment and, in particular, the development of processes for the disposal of industrial waste and the protection of the hydrosphere. The above areas correspond to the goals of UN SDG 6 "Clean Water and Sanitation" and UN SDG 12 "Ensuring sustainable consumption and production patterns" and are included in the concept of a circular economy, which undoubtedly confirms their relevance .
Recently, a significant number of scientific groups have been inclined towards the rejection of traditional inorganic reagents, in favor of materials obtained from plant raw materials (sorbents, ion-exchange materials, etc.). A lot of work has been devoted to the development of technologies for processing industrial and agricultural waste with the production of reagents for water purification processes of waste, as well as a pronounced economic effect due to cost reduction.
It is known that the quality of carbon adsorbents largely depends on the raw materials used . For example, materials obtained from coconut bark, seeds of various plants and other agricultural wastes have proven to be good sorbents , .
Macadamia fruits are hard and very strong spherical drupes, usually 1.5-2 cm in diameter with 1-2 kernels (seeds) inside, because of the original taste of their kernels, are ubiquitous, despite the rather high cost, Increasingly growing interest . In 2019, the global production of macadamia nut kernels amounted to 59 thousand tons, or less than 8% of the pulp of other nuts, such as almonds, walnuts, cashews, pistachios, etc. However, between 2009 and 2019, the production of macadamia nuts grew by 57%, faster than any other tree nut . In Myanmar, macadamia has been cultivated for more than a decade, and the main product is not the kernel, but the nut in the shell (OSM). By 2019, Myanmar is estimated to have produced 300–500 tonnes per year, or less than one percent of global production , , . The production of marketable products (nuts, pulp, concentrates) is accompanied by the formation of a huge amount of waste in the form of shells.
The main purpose of this work is to assess the possibility of using Makadam nutshells as a precursor for the production of carbon adsorbents, as well as to assess the sorption efficiency of the obtained materials.
2. Experimental part
Figure 1 - Ensemble of fragments of prepared raw materials:
a - raw materials; b - sealed containers
Table 1 - Some Evaluated Characteristics of Carbonates (K) and Activated Carbons (AC) Obtained under Rational Conditions for Processing Dense Plant Waste in Myanmar
Adsorbent | Raw Material Yield, % mass. | I, mg/g | МB, mg/g | VΣ, cm3/g | Vs (cm3/g) by vapour: | ||
С6Н6 | CCl4 | H2O | |||||
CS (К) | 25.3 | 403 | 53 | 0.32 | 0.16 | 0.03 | 0.15 |
СS (АC) | 15.2 | 620 | 281 | 0.8 | 0.37 | 0.24 | 0.36 |
PS (К) | 31.0 | 108 | 40 | 0.56 | 0.07 | 0.11 | 0.07 |
PS (АC) | 25.3 | 868 | 152 | 0.92 | 0.37 | 0.34 | 0.19 |
MNS (К) | 55.3 | 864 | 230 | 0.56 | 0.02 | 0.03 | 0.19 |
MNS (АC) | 25.5 | 1183 | 269 | 1.23 | 0.46 | 0.37 | 0.47 |
Note: CS – coconut shell; PS – plum seed; MNS – macadamia net shell
The obtained samples of adsorbents were tested in the processes of capturing vapors of volatile organic solvents (VOS) from their vapor-air mixtures (using the example of n-butanol vapors) . Activated carbons obtained from coconut shells (CS) and plum seeds (PS) were used as a comparison sample . In order to compare the expediency of using activated carbon obtained from MNS to extract vapors of volatile organic solvents from their VOS under almost identical conditions, the kinetics and equilibrium of these processes were studied using the examples of the use of n-butanol vapors and the characterized laboratory-made active carbons.
3. Experimental results and discussion
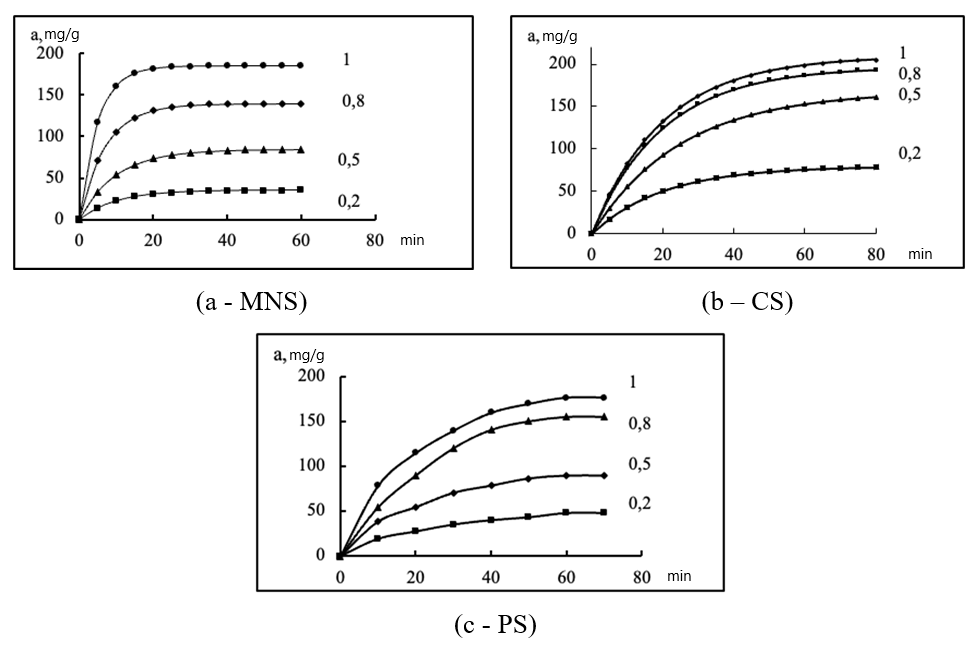
Figure 2 - Kinetics of adsorption at 20 °C of n-butanol vapors from their AVM by samples of used activated carbons (specific consumption of AVM is 2.5 l/(cm2·min), the size of adsorbent grains is 3.0–5.0 mm, the numbers of the curves are the values of p/ps:
а - MNS; b – СS; c - PS
Table 2 - Values of the coefficients A and B of the equation а = А(1-e-В·τ)
Activated carbon | Values of the coefficients A (numerator) and B (denominator) at P/Ps | |||
0.2 | 0,5 | 0,8 | 1,0 | |
CS | 80/0.05 | 167/0.04 | 197/0.05 | 208/0.05 |
MNS | 35/0.10 | 85/0.10 | 140/0.14 | 185/0.20 |
PS | 79/0.05 | 123/0.05 | 158/0.05 | 175/0.05 |
The characterized information allows us to state the difference in the shape of the presented kinetic curves. In the region of low P/PS values, MNS activated carbon demonstrates a lower absorption capacity than PS and CS, which is due to the weak mutual attraction of the molecules of adsorbate and adsorbent MNS. Although the adsorption rate constant B is maximum for activated carbon MNS, at P/Ps = 1, this adsorbent absorbs more n-butanol in its pores than the adsorbent PS, but is inferior to active carbon CS.
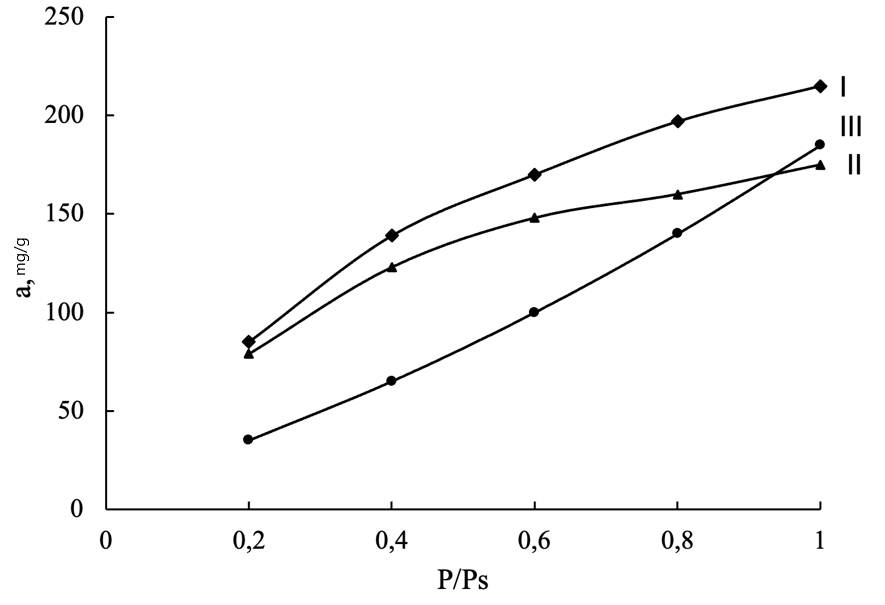
Figure 3 - Adsorption equilibrium of n-butanol on the compared active carbons:
I - CS; II - MNS; III - Ps
Table 3 - Values of equation coefficients а = K(P/Ps)1/n
Activated carbon | Coefficient | |
К | n | |
CS | 222 | 0.61 |
PS | 182 | 0.50 |
MNS | 185 | 1.00 |
The highest value of K, which expresses the absorption capacity of the adsorbent, is observed for activated carbon among the active carbons of plant waste from Myanmar from a large volume of micropores. The parameter n, which characterizes the energy of interaction of coals with butanol, is the smallest for active carbon PS. This corresponds to the most convex adsorption isotherm (Fig. 3), which is favorable for capturing low concentrations of butanol vapors. In terms of value n, the resulting new macadamia nutshell activated carbon is comparable to other types of macadamia nutshell activated carbons.
4. Conclusion
Thus, the studies allow us to state quite satisfactory absorption properties of the obtained new activated carbon from macadamia nutshells, in the studied process of extracting n-butanol vapors from their mixtures with air, which indicates the probable competitiveness of this adsorbent in solving the problems of purification of organic substances from vapors of emissions of high concentrations, provided that its production is organized in the conditions of Myanmar.
