PURIFICATION OF WATER CONTAMINATED WITH PETROLEUM HYDROCARBONS BY USING THE SOLAR PHOTOCATALYTIC METHOD
ОЧИСТКА ВОДЫ, ЗАГРЯЗНЕННОЙ НЕФТЯНЫМИ УГЛЕВОДОРОДАМИ, СОЛНЕЧНЫМ ФОТОКАТАЛИТИЧЕСКИМ МЕТОДОМ
Научная статья
Хамиди Хамед1, Ниазманд Милад Ахмад2, Асрар Канишка3, *, Окольникова Г.E.4
1 ORCID: 0000-0001-7529-9711;
2 ORCID: 0000-0003-3590-3157;
3 ORCID: 0000-0003-1223-4122;
4 ORCID: 0000-0002-8143-4614;
1 Джаузджанский университет, Джаузджан, Афганистан;
2, 3, 4 Российский университет дружбы народов (РУДН), Москва, Россия
* Корреспондирующий автор (kanshkas[at]mail.ru)
АннотацияВ этой статье изучалась обработка синтетических стоков, содержащих углеводороды нефти, с использованием солнечного фотокаталитического процесса с наночастицами TiO2, нанесенными на бетонные плиты. Назначенный солнечный фотореактор включал в себя 40-литровый резервуар для хранения загрязняющих веществ с погружным насосом, ступенчатую систему, состоящую из четырех ступеней, которые были стабилизированы фотокатализатором TiO2, перелив объемом 4 литра наверху лестницы и металлическое шасси с принадлежностями. В этом реакторе ультрафиолетовая (УФ) часть солнечного света использовалась в качестве источника излучения вместо УФ-ламп. Эффективность этой системы в оптимальных условиях оценивается при pH равном 4, массовой загрузке TiO2 80 г / м2, продолжительности УФ-облучения 240 мин, начальной концентрации 100 мг / л и концентрации H2O2 2000 мг. / Л соответствует 77,35% удалению химической потребности в кислороде (ХПК), 79,12% удалению всех нефтяных углеводородов (TPH) и 86,91% удалению полициклических ароматических углеводородов (PHA). Результаты испытаний газовой хроматографии / пламенно-ионизационного детектирования (GC-FID) отработанного образца до и после обработки и в оптимальных условиях показали удаление большей части полиароматических углеводородов, а то, что осталось, было безопасными алифатическими углеводородами.
Ключевые слова: нефтяные углеводороды, солнечный реактор, диоксид титана, бетон.
PURIFICATION OF WATER CONTAMINATED WITH PETROLEUM HYDROCARBONS BY USING THE SOLAR PHOTOCATALYTIC METHOD
Research article
Hamidi Hamed1, Niazmand Milad Ahmad2, Asrar Kanishka3, *, Okolnikova G.E.4
1 ORCID: 0000-0001-7529-9711;
2 ORCID: 0000-0003-3590-3157;
3 ORCID: 0000-0003-1223-4122;
4 ORCID: 0000-0002-8143-4614;
1 Jawzjan University, Jawzjan, Afghanistan;
2, 3, 4 People's Friendship University of Russia (RUDN University), Moscow, Russia
* Corresponding author (kanshkas[at]mail.ru)
AbstractThis paper studied synthetic effluents' treatment containing petroleum hydrocarbons using a solar photocatalytic process with TiO2 nanoparticles coated on concrete plates. The assigned solar photoreactor included a 40-liter contaminant storage tank with a submersible pump, a stair system including four steps which was stabilized with TiO2 photocatalyst, an overflow with 4 liters volume at the top of the stairs, and metal chassis with its accessories. In this reactor, the ultraviolet (UV) part of sunlight was used as the radiation source instead of UV lamps. The efficiency of this system in optimal conditions evaluated as at pH equal to 4, mass loading of TiO2 equal to 80 g/m2, duration of UV irradiation equal to 240 min, initial concentration 100 mg/L, and concentration of H2O2 at 2000 mg/L equivalent to 77.35% removal of chemical oxygen demand (COD), 79.12% removal of total petroleum hydrocarbons (TPH), and 86.91% removal of polycyclic aromatic hydrocarbons (PHAs). The gas chromatography/flame ionization detection (GC-FID) test results of the effluent sample before and after treatment and under optimal conditions indicated removing most of the polyaromatic hydrocarbons and what remained was safe aliphatic hydrocarbons.
Keywords: petroleum hydrocarbons, solar reactor, Titanium dioxide, concrete.
IntroductionPetroleum and its fractions are a collection of aliphatic and aromatic hydrocarbons. The latter is a stable, environmentally persistent, toxic, mutagenic, and carcinogenic compound [1], [2], [3]. Sources of oil pollution in the aquatic environment include oil spill, refinery effluent, water produced in oil fields, leaks from storage tanks or oil pipelines, ballast water, effluent from car wash, construction materials and, repairing and runoff resulted from rains in pump stations or washing inner-city tunnels [4], [6], [9]. In Afghanistan, oil wells are primarily located in the Afghan-Tajik and the Amu Darya basins [10] in the north of the country (Sar-e-Pul, Faryab Balkh). Simultaneously, the refinery and storage facilities are situated in the Balkh, Herat, and Sare Pul provinces. In the north of Afghanistan, extensive operations to exploit and transport petroleum products, the location of oil and gas facilities near the water sources and, lack of awareness about the causes and effects of oil pollution have increased the potential risk of contamination of water resources with petroleum compounds.
The methods for treatment of water and wastewater contaminated with petroleum compounds include physical, chemical, biological, and combined processes such as adsorption [11], filtration [12], membrane [13], electrochemical coagulation [14], advanced oxidation [15], stabilization ponds [16], anaerobic [17], flotation [18] etc. Moreover, each of these methods has advantages and disadvantages. These methods' efficiency is relevant to the level of treatment, the environment, and facility for application, capital cost, energy costs, technological simplicity, and maintenance. Among these methods, one of the newest, efficient, and most practical chemical methods is advanced oxidation, such as the heterogeneous photocatalytic process. This method uses sunlight to remove different harmful bacterias and organic pollutants with the assistance of a concrete photocatalyst. The photocatalytic process is mainly related to solar energy use, especially photocatalysis, and emphasizes converting solar energy into chemical energy[19]. In this process, complex and hardly-biodegradable organic compounds in the suspended or stabilized form and with the presence of photocatalyst nanoparticles TiO2, expose to UV light and decompose into simpler compounds by producing highly oxidizing hydroxyl radicals [20].
Due to the active feature of the TiO2 near-ultraviolet region, this process is usually done with UV-A lamps [21]. In this research, with a unique look at the treatment method's environmental and economic dimensions, solar energy replaced the lamp. Considering that only 5% of the radiation reaching the earth's surface is UV, of which 98% is UV-A, and the intensity of its radiation near the earth's surface on a sunny day is about 20 to 30 watts per square meter, it can be understood that this part of the sunlight contains a small amount of the subtle radiation [22]. Solar photocatalytic methods have already been used to treat wastewater in different industries such as paper, textile, azo dye, detergent, etc. [23], [24]. Shavisi et al. studied photocatalytic degradation of ammonia in petrochemical wastewater using solar light/TiO2 photocatalysis. They selected the maximum concentration of ammonia in petrochemical wastewater equal to (975 mg/L). As a result, the solar reactor system led to 96.5% removal of ammonia in pH =11 [25]. De Andrade et al. used TiO2 supported on autoclaved cellular concrete. They assessed the performance of three separate systems, TiO2/ACC/UV, TiO2-P25/UV, and UV in the oxidation of indigo carmine involving liquid chromatography combined with mass spectrometry. The results indicated that TiO2-P25/UV and UV systems displayed removal rates of 60%, whereas TiO2/ACC/UV about 100%, after 350 min of exposure [26]. Ghaly et al. studied the solar photocatalytic degradation of paper mill wastewater over synthesized nano TiO2. As a result, TiO2 optimum dose of 0.75 g/L and pH value of 6.5, with 75% COD removal of the wastewater and 80% reduction of total suspended solids, was achieved within 180 min solar irradiation time [27]. Souza et al. employed photocatalysis to treat textile effluent from industrial laundry jeans and, for this purpose, used catalysts TiO2 P25, TiO2, ZnO and Nb2O5, under artificial UV irradiation. The result based on textile effluent at pH 3.0 and catalysts concentration of 0.250 g L−1 indicated 95.91%; 87.35%; 86.95%, and 59.18% of reduction after 300 min of artificial irradiation.
TiO2 was responsible for reducing 70 % of COD, and the photocatalytic activity of both TiO2 and TiO2 P25 was very close to each other [28]. The purpose of this study was to use the ultraviolet part of sunlight instead of lamps in the photocatalytic process to save energy and prevent the harmful environmental hazards caused by the production of electricity from other conventional methods.
Materials and Methods. Photoreactor specifications
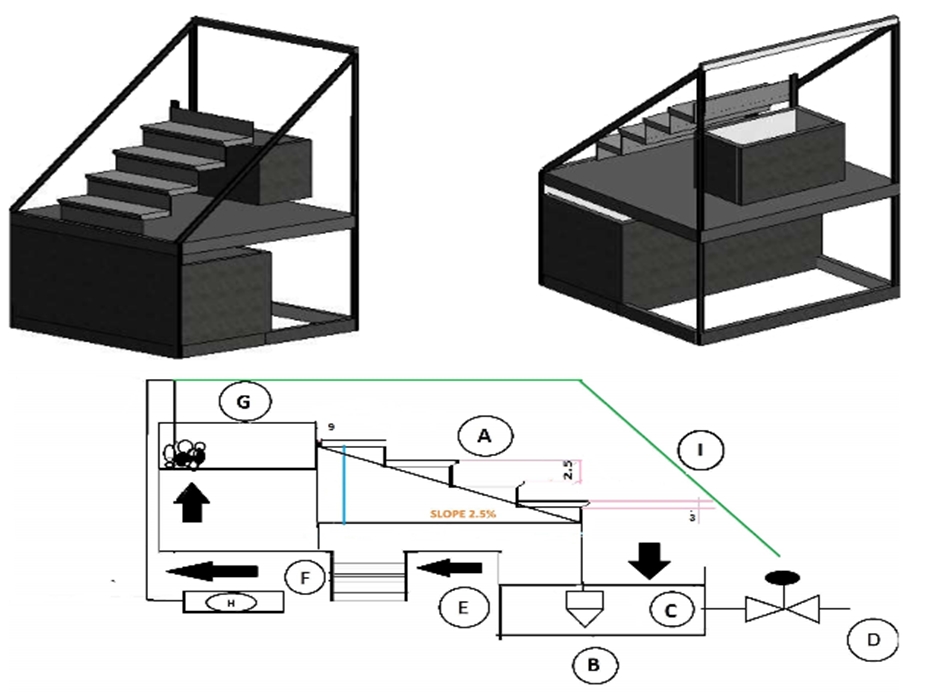
In this research, the Manning equation was used for the hydraulic design of the photoreactor-stepped structure. For the thickness of 1.5 mm of effluent, the laminar flow's maximum flow rate was considered 3 liters per min. The photoreactor was built with a continuous rotational flow regime. It consisted of three parts: the contaminant storage tank, the photocatalytic reactor core, and the chassis and connected accessories. The photoreactor's stepped body was made from lightweight concrete with pumice aggregate fixed with TiO2 nanoparticles and placed at vertical distances of 2.5 cm. It consisted of four steps with dimensions of 3x9x18 cm and had a slope of 2.5 %. A galvanized sheet overflow with 4 liters volume steadily directed the flow on the concrete stairs' surface on the top of the concrete stairs.
Furthermore, to protect the reactor from wind, rain and prevent evaporation, it was covered with ordinary glass plates with a thickness of 3 mm and an ultraviolet light transmission coefficient of 70%. The 40-liter contaminant storage tank, which was equipped with a submersible aquarium pump, circulated the effluent at a rate of 200 liters per hour. An aeration pump with 250 liters per hour speed was placed in the overflow to compensate for the effluent's dissolved oxygen.
Fig. 1 – The design (scheme) of the solar photoreactor:
A – Concrete stairs; B – supply tank; C – pump; D – sampling valve; E – thermostatic bath; F – connection tube; G – overflow; H – aeration pump; I – glass layer (3mm)
Concrete structure and nanoparticle covering
The wooden molds with dimensions of 4x12x24 cm were used to make concrete slabs by the preplaced aggregate method.
In this method, first, the mold was filled with coarse pumice stone، then cement-sand slurry with a ratio of 1:1 was added and placed for three days to attain concrete.
Table 1 – The materials and mixing ratios used in the concrete structure
| Cement | Water | Pumice | Sand | Porosity, % |
| Type 2, volume 300 KG | 150 liters drinking water | 10-15 mm 500 Kg | 2-5 mm 500 Kg | 20 (based on ASTMD 7063 standard) |
In addition, the TiO2 nanoparticle powder used in this process had a purity of 99.9% and consisted of two phases of anatase, 78.8%, and rutile 21%. Besides, the slurry method was used to stabilize nanoparticles.
The effluent simple
The studied effluent was prepared synthetically from a combination of distilled water and diesel of Hairatan refinery in different concentrations of 100, 200, 300, 400, and 500 mg/l.
These concentrations had COD equal to 162, 265, 382, 484 and 597 ppm, TPH equal to 33, 82, 135, 189 and 229 ppm, and PAHs equal to 36, 81, 129, 133,169, 204 ppb.
The measurement of parameters
The solar photoreactor was set under direct angel of sunlight in 36°42'42.7"N 67°12'55.4"E and elevation of 357 m from the sea level. Various measuring devices have been used to analyze and measure the necessary parameters (Table 2).
Table 2 – The parameters and devices used for identifying the optimal condition
| Parameter | Tool (the device used for measuring) |
| Sunlight intensity | Lutron UVA-365 UV Light Meter (radiometer). Relevant information was recorded for 30 min from 9 am to 6 pm for four months. |
| Analyzing and measuring effluent samples of total petroleum hydrocarbon (TPH) before and after the process based on ASTM D7066-04 standard | InfraCal 2 TOG/TPH Analyzer of IndiaMART |
| Measuring polycyclic aromatic hydrocarbon (PAHs) based on EPA 8100 standard | HP GC-FID 5890 Series ll |
| Measuring chemical oxygen demand (COD) based on 5220-B standard | Hach reactor |
| pH adjustment | Hydrochloric acid (HCI) |
Titanium dioxide (TiO2)
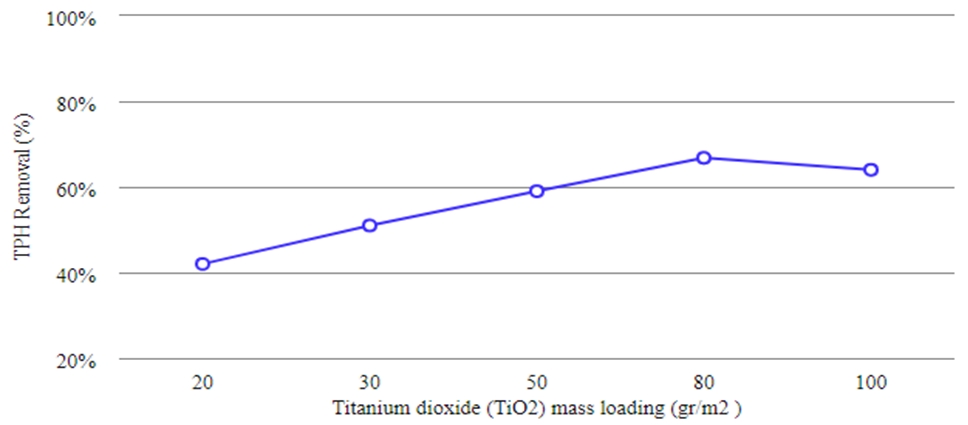
Among the concrete slabs with 20, 30, 50, 80, and 100 g/m2 titanium dioxide (TiO2) loading, 80 g loading amount had the highest removal efficiency of 66.8% for the total petroleum removal, therefore selected as the optimal value.
Fig. 2 – The impact of TiO2 on the removal of TPH
pH level
The highest removal efficiency was observed at an acidic pH of about 4 with 65% removal of TPH. Therefore, pH 4 was the optimal value for the purification of effluent contaminated with petroleum in the photocatalytic process.
The optimal system condition
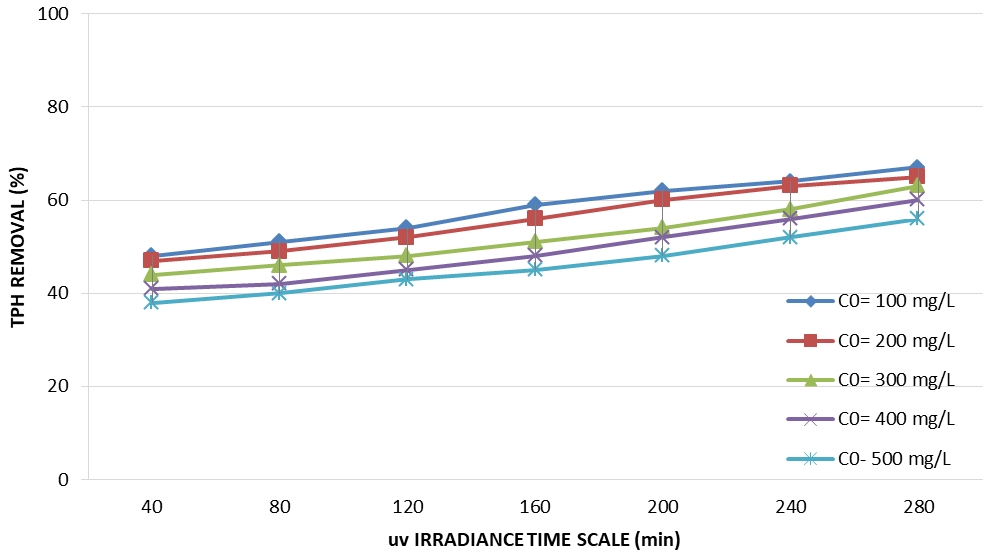
By examining the input concentration of pollutants from 100 to 500 mg / L for four months (May, June, July, August) and from 9:00 am to 6:00 pm, the maximum removal efficiency for the lowest input concentration (100 mg/L) after 280 min of UV sunlight was 67% (Figure 3). It was observed that with increasing the concentration of contaminants, the efficiency of the system decreases. The reduction in efficiency with increasing contamination was the decrease in the absorption of light photons, the photocatalyst surface's saturation due to the adsorption of the contaminant's and the inhibition of the reaction between the pollutant hub molecule and hydroxyl radicals.
Fig. 3 – The intensity of the sun's UV and percentage of the removal efficiency
Launching photoreactor under optimal conditionFinally, after identifying the optimal condition for any parameter, the reactor has been relaunched under pH 4, TiO2 loading amount of 80g, input density (effluent simple) 100 mg/L, and H2O2 density of 2000 mg/L. The highest removal efficiency for PAH was 87% under 240 min UV, TPH 79% under 240 min UV, and COD 77% under 280 min UV irradiation. UV at 240 min was the optimal irradiation for removing petroleum hydrocarbons from contaminated water. The GC-FID test was used on both primary effluent and purified water ( treated water after using the photocatalytic method), which indicated a high percentage removal of PAH, TRH, and COD (Figure 4).
Fig. 4 – The removal efficiency of photoreactor under optimal condition
ConclusionDifferent studies have already indicated that using the UV part of sunlight instead of UA-V lamp can significantly remove hazardous petroleum hydrocarbons from polluted water and wastewater. The current study results showed that at low-level concentrations under the UV irradiation time equal to 240 minutes (6 days of starting the solar reactor), petroleum pollution could meet the required standards of discharge and purification of polluted water.
It is expected that using solar photocatalytic reactors can be practically and economically beneficial for the treatment of effluents from refineries and oil fields in countries such as Afghanistan, where there is intense sunlight for more than 300 days a year. Also, the stabilization of the photocatalyst on the concrete surface, which is an essential element in the structures of water and wastewater treatment plants, significantly increases the efficiency of this method on an industrial scale.
| Конфликт интересов Не указан. | Conflict of Interest None declared. |
Список литературы / References
- Hussein I. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation / Hussein I Abdel-Shafy, Mona S.M. Mansour. // Egyptian Journal of Petroleum. 2016. No 25. – P. 107-123.
- Harris K.L. Bioaccessibility of polycyclic aromatic hydrocarbons: relevance to toxicity and carcinogenesis / Harris K.L, Banks L.D, Mantey J.A. et al. // Expert Opin Drug Metab Toxicol. 2013. No 9. – P. 1465-1480.
- Stepnowski P. Enhanced photo degradation of contaminants in petroleum refinery waste water / Stepnowski P, Siedlecka E.M., Behrend P. et al. // Water research. 2002. No 36. – P. 288-294.
- Zhang B.Y. Marine Oil Spills -Oil Pollution, Sources and Effects. World Seas: An Environmental Evaluation / Zhang B.Y., Matchinski E.J., Chen B. et al. // Ecological Issues and Environmental Impacts No 3. – P. 391-406.
- Saien J. Organic Pollutants Removal from Petroleum Refinery Wastewater with Nanotitania Photocatalyst and UV Light Emission / Saien J, Shahrezaei F. // International Journal of Photoenergy. No 1. – P. 1-5.
- Bakke T. Environmental impacts of produced water and drilling waste discharges from the Norwegian offshore petroleum industry / Bakke T, Klungsøyr J, Sanni S. // Marine Environmental Research. 2013. No 92. – P. 154-169.
- Werschkun Emerging risks from ballast water treatment: The run-up to the International Ballast Water Management Convention / Werschkun B, Banerji S. // Chemosphere. 2014. No 112. – P. 256-266
- Tekere M. An assessment of the physicochemical properties and toxicity potential of carwash effluents from professional carwash outlets in Gauteng Province, South Africa / Tekere, M, Sibanda T, Maphangwa K.W. // Environmental Science and Pollution Research. 2016. No – P. 11876–11884.
- Wang S. The Harm of Petroleum-Polluted Soil and its Remediation Research / Wang S, Xu Y, Lin Z. et al. // AIP Conference Proceedings. 2017. No 1864. – P. 1-9.
- Mehrad T. Assessment of oil and gas resources of northern Afghanistan and their impact on energy security in the country / Mehrad A.T., Zvolinski V.P., Kapralova D.O. et al. // IOP Conference Series: Materials Science and Engineering. 2020. No 976. – P. 1-8.
- Ali I. Advances in water treatment by adsorption technology / Ali I, Gupta V.K. // Nature Protocols. 2007. No 1.- 2661-2667.
- Pooi, C.K. Review of low-cost point-of-use water treatment systems for developing communities / Pooi C.K., Ng H.Y. // Clean Water. No 1. –P. 1-8.
- Lee A. Darling SB Membrane materials for water purification: design, development, and application / Lee A, Elam J.W. // Environmental Science: Water Research & Technology. 2016. No 2.- P. 17-42.
- Guohua C. Electrochemical technologies in wastewater treatment / Guohua C. // Separation and Purification Technology No 38. – P. 11–41.
- Mehmet A.O. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review / Mehmet A.O., Jean J.A. // Critical Reviews in Environmental Science and Technology. 2014. No 44. – P. 2577–2641.
- Dalu J.M. Duckweed based wastewater stabilization ponds for wastewater treatment (a low cost technology for small urban areas in Zimbabwe) Dalu J.M, Ndamba J. // Physics and Chemistry of the Earth. 2003. No 28. – P. 1147-1160.
- Ozgun H. A review of anaerobic membrane bioreactors for municipal wastewater treatment: Integration options, limitations and expectations / Ozgun H, Dereli R.K., Ersahin M.E. et al. // Separation and Purification Technology. 2013. No 118. – P. 89-104.
- Rubio J. Overview of flotation as a wastewater treatment technique / Rubio J, Souza M.L., Smith R.W. // Minerals Engineering. 2002. No 15. – P. 139-155.
- Dong S. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: a review / Dong S, Feng J, Fan M. et al. // RSC Advances. 2015. No 5. – P. 14610-14630.
- Pirhashemi M. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts / Pirhashemi M, Habibi Y.H., Rahim P.S. // Journal of Industrial and Engineering Chemistry. 2018. No 62. – P. 1-25.
- Lee S.Y. TiO2 photocatalyst for water treatment applications / Lee S.Y., Park S.J. // Journal of Industrial and Engineering Chemistry. 2013. No 19. – P.1761-1769.
- Lazar M.A. Photocatalytic Water Treatment by Titanium Dioxide: Recent Updates / Lazar M.A., Varghese S.; Nair S.S. // Catalysts 2012. No 2. – P. 572-601.
- Didier R. Solar photocatalysis: a clean process for water detoxification / Didier R, Malato S. // Science of The Total Environment. 2002. No 291. – P. 85-97.
- Zhang T. Recent Progress in TiO2-Mediated Solar Photocatalysis for Industrial Wastewater Treatment / Zhang T, Wang S, Zhang X. // International Journal of Photoenergy. 2014. No 1. – P. 1-12.
- Shavisi Y. Application of solar light for degradation of ammonia in petrochemical wastewater by a floating TiO2/LECA photocatalyst / Shavisi Y, Sharifinia S, Zendehzaban M. et al. // Journal of Industrial and Engineering Chemistry. 2013. No 5. – P. 2806-2813.
- De Andrade F.V. A novel TiO2/autoclaved cellular concrete composite: From a precast building material to a new floating photocatalyst for degradation of organic water contaminants / De Andrade F.V., De Lima G.M., Augusti R. et al. // Journal of Water Process Engineering. 2015. No 7. – P. 27-35.
- Ghaly Y.M. Treatment of highly polluted paper mill wastewater by solar photocatalytic oxidation with synthesized nano TiO2 / Ghaly Y.M., Jamil T.S., El-Seesy I.E. et al. // Chemical Engineering Journal. 2011. No 168. – P. 446-454.
- Souza R.P. Photocatalytic activity of TiO2, ZnO and Nb2O5 applied to degradation of textile wastewater / Souza R.P., Freitas TKFS, Domingues F.S. et al. // Journal of Photochemistry and Photobiology A: Chemistry. 2016. No 329. – P. 9-17.