RESULTS OF LABORATORY STUDIES OF THE EFFECT OF AN ELECTRIC FIELD ON THE ICE-FORMING PROPERTIES OF THE AD-1 PYROTECHNIC COMPOSITION (translation of the original publication in English)
RESULTS OF LABORATORY STUDIES OF THE EFFECT OF AN ELECTRIC FIELD ON THE ICE-FORMING PROPERTIES OF THE AD-1 PYROTECHNIC COMPOSITION (translation of the original publication in English)
Abstract
Translation of the original publication Геккиева С.О. Результаты лабораторных исследований влияния электрического поля на льдообразующие свойства пиротехнического состава АД-1 / С.О. Геккиева // Международный научно-исследовательский журнал. — 2025. — № 10 (160). — URL: https://research-journal.org/archive/10-160-2025-october/10.60797/IRJ.2025.160.84 (дата обращения: 08.11.2025). — DOI: 10.60797/IRJ.2025.160.84.
This article presents the equipment, methodology, and results of laboratory studies on the effect of an electric field on the ice-forming properties of the highly effective ice-forming composition "AD-1" with 8% AgI content, which is used to equip third-generation anti-hail complexes of the "Alazan" family. A special set of equipment was created for conducting laboratory experiments, and a method of charging reagent particles in the electric field of a flat capacitor was used.
The results of tests on the ice-forming component AD-1 in the presence of an electric field are presented. According to the conducted research, the presence of an electric field reduces the yield of ice-forming particles across the entire range of accepted temperatures, from minus 3°C to minus 12°C. It was found that the specific yield of ice-forming nuclei decreases threefold when the electric field strength increases from 100 to 300 V/cm.
In experiments without an electric field, on the contrary, an increase in ice-forming particles is observed, with maximum yield in the temperature range from minus 11°C to minus 12°C, followed by a further decrease and a sharp jump at minus 14°C.
1. Introduction
It is known that electrical phenomena accompanying thermodynamically irreversible phase transitions in clouds are accompanied by intense electrification of the surface of both the reactants and the resulting particles. There is reason to believe that their role is significant , , . The increase in electrical activity in convective clouds after exposure to the ice-forming crystallizing reagent AgI is analyzed in , , . The analysis demonstrates an increase in electrical activity in clouds after active exposure based on the frequency of lightning discharges, which was calculated from the radar characteristics of the clouds under study. Reagents and technical means for active exposure (AE) are currently being improved , , , . It was noted that when conducting AW on cloud systems, the presence of an atmospheric electric field and charge on reagent particles can affect the specific yield of ice-forming nuclei. This article presents an analysis of the laboratory results.
2. Research methods and principles
A set of equipment was created to study the influence of an electric field on the ice-forming properties of the AD-1 pyrotechnic composition and its ice-forming properties. The yield of active particles A per unit mass of the substance converted into an aerosol (g-1) after the reagent is released is determined by the formula:
where:
n — the number of ice crystals in the microscope's field of view;
V — the volume of the aerosol chamber, cm³;
S — the floor area of the cloud chamber, cm²;
s — the area of the microscope's field of view, cm²;
v — the volume of the aerosol sample, cm³;
m — the mass of the substance converted into an aerosol, g.
The theoretical yield of active particles for the most active ice-forming substance, silver iodide, at the lowest measurement temperature of minus 25°C, equal to 1019 g-1, can be considered as an upper limit.
3. Main results
The experiments were conducted in a sublimation chamber with an electric field strength of 300 V/cm, as well as without a field. The experimental results are presented in Tables 1–2 and Figure 1.
Table 1 - Specific yield of crystals in the presence of an electric field
Distance between plates, m | Temperature in the chamber, °С | Reagent mass, g
| Voltage, kV | Specific yield, ×1012g-1
|
0,04 | –14,0 | 0,01 | 9,0 | 2,4 |
0,04 | –13,5 | 0,01 | 5,0 | 2,3 |
0,04 | –13,0 | 0,01 | 5,0 | 2,6 |
0,04 | –12,5 | 0,01 | 5,0 | 2,3 |
0,04 | –12,0 | 0,01 | 2,5 | 2,6 |
0,04 | –11,5 | 0,01 | 5,0 | 2,9 |
0,04 | –11,0 | 0,01 | 5,0 | 2,2 |
0,04 | –10,5 | 0,01 | 9,0 | 2,1 |
0,04 | –10,0 | 0,01 | 5,0 | 2,3 |
Table 2 - Specific yield of crystals in the absence of an electric field
Temperature in the chamber, °С | Reagent mass, g
| Specific yield, ×1012g-1
|
–14,0 | 0,01 | 4,1 |
–13,5 | 0,01 | 4,0 |
–13,0 | 0,01 | 3,0 |
–12,5 | 0,01 | 2,8 |
–12,0 | 0,01 | 3,6 |
–11,5 | 0,01 | 3,8 |
–11,0 | 0,01 | 3,0 |
–10,5 | 0,01 | 2,0 |
–10,0 | 0,01 | 2,1 |
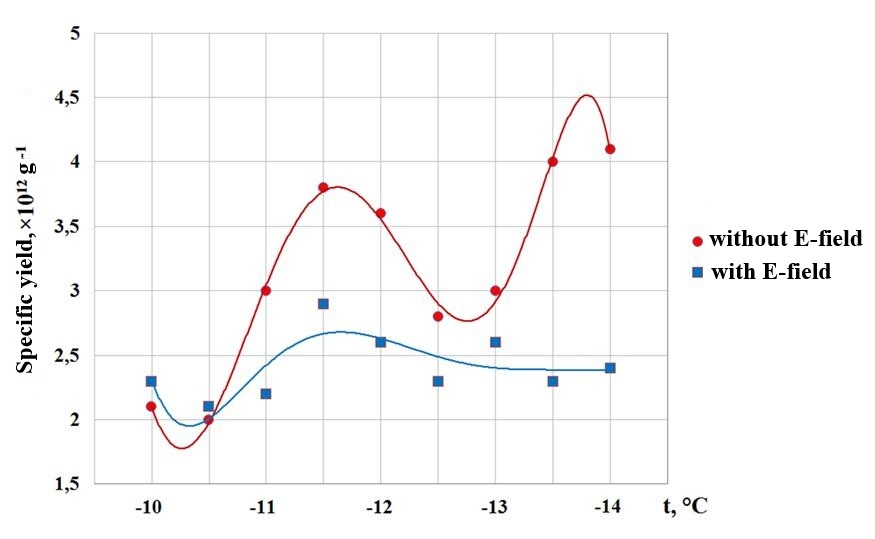
Figure 1 - Dependence of the specific yield of crystals on temperature in the presence and absence of an electric field during reagent sublimation
The graph shows that in experiments without an electric field, the maximum yield of ice-forming particles is achieved in the temperature range from -11 to -12°C, with a subsequent decrease and a sharp peak at -14°C. In experiments with an electric field, the specific yield of crystals does not exhibit any clearly defined maxima. The data presented above indicate that the presence of an electric field weakens the ice-forming properties of the experimental material. When the reagent was sublimated in an electric field generated by a unipolar rectifier, the reagent flow was deflected primarily toward the grounded plate (Figure 2).
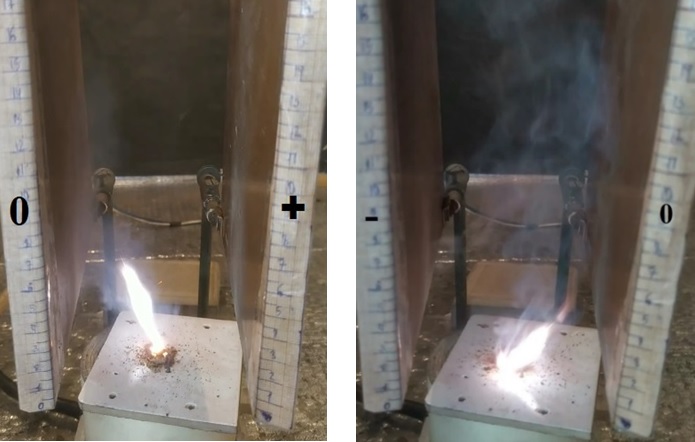
Figure 2 - Reagent particle flow deflection during sublimation using a unipolar rectifier

Figure 3 - Deviation of the flow of reagent particles during sublimation in an electric field
The results of laboratory experiments to determine the specific yield of crystals in the presence of an electric field are presented in Table 3.
Table 3 - Value of the specific yield of crystals in the presence of an electric field
Tension, V/cm | Temperature in the chamber, °С | Reagent mass, g
| Average number of crystals per frame | Specific yield, ×1011g-1
|
300 | -8,0 | 0,21 | 106 | 0,63 |
297 | -9,0 | 0,21 | 35 | 0,21 |
290 | -8,0 | 0,21 | 169 | 1,00 |
293 | -7,4 | 0,21 | 255 | 1,50 |
270 | -11,2 | 0,18 | 19 | 0,13 |
270 | -10,5 | 0,21 | 1009 | 6,00 |
233 | -9,9 | 0,20 | 603 | 3,80 |
233 | -9,9 | 0,21 | 173 | 1,03 |
233 | -7,7 | 0,21 | 1112 | 6,60 |
233 | -7,3 | 0,21 | 58 | 0,35 |
233 | -6,5 | 0,21 | 178 | 1,60 |
233 | -5,2 | 0,19 | 71 | 0,46 |
100 | -7,8 | 0,21 | 493 | 2,90 |
90 | -8,6 | 0,21 | 876 | 5,20 |
90 | -7,7 | 0,21 | 544 | 3,20 |
Figure 4 shows the dependence of the specific crystal yield on the electric field strength. It can be seen that as the electric field strength increases, the specific crystal yield tends to decrease, and at a fairly rapid rate.
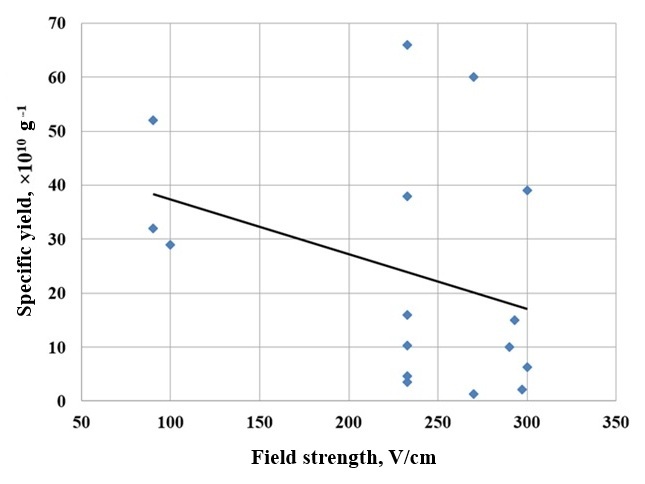
Figure 4 - Dependence of the specific yield of crystals on the electric field strength
A = – 0,1E + 47,5,
where A is the specific yield of ice-forming nuclei, g-1;
E is the electric field strength, V/cm.
As can be seen from the equation, the specific yield of crystals decreases with increasing electric field strength. It is known that the electrical effect that occurs during crystallization depends significantly on the chemical composition of cloud water. In real conditions, cloud water typically contains dissolved acids, alkalis, and salts. Depending on their content, the electrification effect of crystallizing droplets can either increase or decrease. Experiments were similarly conducted in the absence of an electric field.
Table 4 - Value of the specific yield of crystals in the absence of an electric field
Temperature in the chamber, °С | Reagent mass, g
| Average number of crystals per frame | Specific yield, ×1011g-1
|
-10,6 | 0,21 | 1115 | 6,6 |
-10,6 | 0,21 | 585 | 3,5 |
-10,5 | 0,21 | 1016 | 6,0 |
-9,9 | 0,21 | 617 | 3,7 |
-9,9 | 0,21 | 686 | 4,1 |
-9,7 | 0,21 | 436 | 2,6 |
-9,7 | 0,21 | 2087 | 12,0 |
-9,5 | 0,21 | 263 | 1,6 |
-9,4 | 0,21 | 909 | 5,4 |
-9,3 | 0,21 | 432 | 2,6 |
-8,6 | 0,21 | 286 | 1,7 |
-8,5 | 0,21 | 338 | 2,0 |
-8,2 | 0,21 | 1348 | 8,0 |
-7,9 | 0,21 | 1134 | 6,8 |
An analysis of the results presented in Tables 3–4 shows that the specific yield of ice-forming nuclei at an electric field strength of 100 V/cm at temperatures of minus 6–7°C decreases by a factor of 1,5. As the electric field strength increases from 233 to 270 V/cm at temperatures of minus 7–8°C, the specific yield of ice-forming nuclei decreases by a factor of 2. As the electric field strength increases from 270 to 300 V/cm at temperatures of minus 8–9°C, the specific yield of ice-forming nuclei decreases by a factor of 3. It follows from the above that during sublimation of the experimental material under the influence of an electric field, the specific yield of ice-forming particles decreases even with decreasing temperature.
4. Conclusion
Equipment and methodology have been developed to study the influence of an electric field on the ice-forming efficiency of the AD-1 pyrotechnic composition. Based on an analysis of the obtained results, it was found that with increasing electric field strength, the specific yield of crystals decreases, which is likely due to the influence of the electric field on condensation and coagulation processes. The dependence of the specific yield of ice-forming particles of the experimental material on electric field strength at different temperatures was determined. In anti-hail applications, the reagent is introduced into the cloud layer between isotherms of -6 to -10°C. The presence of an electric field at this level can weaken the ice-forming properties of the introduced reagent and, consequently, lead to a certain decrease in the concentration of ice-forming particles. Therefore, analyzing and considering the influence of various cloud parameters on the effectiveness of crystallizing reagents will facilitate the development of more effective pyrotechnic compositions and will also influence the correct dosage of the introduced reagent and the overall consumption to achieve maximum effect, depending on the stated project goals.
