ИНДУКЦИЯ АПОПТОЗА В КЛЕТКАХ МЕЛАНОМЫ КОЖИ КАТИОННЫМИ ПЕПТИДАМИ
Лушникова А.А.1, Понкратова Д.А.2, Морозова Л.Ф.3, Андреев С.М.4
1ORCID:0000-0002-7838-1005, Доктор биологических наук, 2Кандидат биологических наук 3Кандидат медицинских наук, ФБГУ «Российский онкологический научный центр им.Н.Н.Блохина» Минздрава России, Москва. 4Кандидат химических наук , ФГБУ «ГНЦ Институт иммунологии»ФМБА России, Москва
ИНДУКЦИЯ АПОПТОЗА В КЛЕТКАХ МЕЛАНОМЫ КОЖИ КАТИОННЫМИ ПЕПТИДАМИ
Аннотация
Меланома кожи (МК) – агрессивное онкологическое заболевание с ранним метастазированием. Диссеминированная МК резистентна к большинству химиотерапевтических средств и в ~ 30% случаев приводит к летальному исходу. Эту проблему позволяет решить молекулярно-направленная терапия. Перспективными мишенями для таргетной терапии считаются шаперонные белки нуклеолин/NCL и нуклеофозмин/NPM, которые формируют около 70% объема ядрышка и гиперэкспрессируются в опухолевых клетках. В работе впервые предложено использовать катионные пептиды ─ возможные лиганды рецепторного NCL─ для индукции апоптоза в культурах клеток меланомы кожи человека.
Ключевые слова: метастатическая меланома кожи, клеточные линии, катионные пептиды, пролиферация клеток, избирательное ингибирование.
Lushnikova A.A.1, Ponkratova D.A.2, Morozova L.F.3, Andreev S.M.4
1ORCID: 0000-0002-7838-1005, MD, 2PhD, 3MD, N.N.Blokhin Cancer Research Center, Moscow, Russia, 4PhD, State Research Center Immunology Institute of Federal Medical-biologic Agency, Moscow, Russia
INDUCTION OF APOPTOSIS IN CUTANEOUS MELANOMA CELLS BY CATIONIC PEPTIDES
Abstract
Cutaneous melanoma (CM) is an aggressive oncological disease with early growth of metastasis. Disseminated MK is resistant to most chemotherapeutic agents with letality in ~ 30% of cases. Molecular-directed therapy allows to solve this problem. Chaperone proteins nucleolin / NCL and nucleophosmin / NPM, which form about 70% of the nucleolus volume, are considered as promising targets for targeted therapy. These proteins are overexpressed in tumor cells. It was first proposed to use cationic peptides – a possible ligands for receptor NCL - for the induction of apoptosis in cutaneous melanoma cell cultures.
Keywords: metastatic cutaneous melanoma, cell lines, cationic peptides, cell proliferation, selective inhibition.
Introduction
Metastatic cutaneous melanoma is one of the prevalent skin cancers which upon the progression has no specific prolonged treatment. Research of diagnostics biomarkers, as well as predictive and prognostic markers is the actual problem. Blockage of the metastasis process also remains a significant clinical challenge. Nucleolin/C23 and nucleophosmin/B23 ─ major nucleolar phosphoproteins which are considered now as the cell proliferations markers and potential targets for melanoma treatment. Moreover, these molecular shaperons control DNA transcription, RNA translation, ribosome biogenesis, chromatin remodeling, cell signaling, differentiation, invasion, angiogenesis and carcinogenesis. Nucleolin is overexpressed on the surface of cancer cells but the expression in normal ones is significantly lower.[1, Р. 689]. Differential expression level of C23 and B23 in tumor and normal cells allows to use these proteins as a target for selective inhibition of tumor growth.
The aim of the study was an analysis of the activity for cationic peptides as anticancer agents.
Materials and Methods
Two original cell lines melIS and melH obtained from the lymph nodes of the patients with metastatic cutaneous melanoma were used. Mutation status was studied by PCR. Primers for exon 15 BRAF gene were: 5′- ACCTAAACTCTTCATAATGCT-3′ (forward) and 5′- ACAACTGTTCAAACTGATGG-3′ (reverse). Normal human fibroblast line H1036 was used as a control. The cells were cultured in standard RPMI 1640 medium supplied with 10% fetal calf serum. Fifteen cationic peptides with different structure, molecular mass from 1090 to 2338 Dalton and molecular charge from 4+ to 16+ were tested by standard MTT cell proliferation assay (MTT viability test). Previously the peptides were synthesized, characterized and patented as low-toxicity compounds that can be used as DNA/RNA carriers in gene therapy based medicines for gene silencing. [2, Р.8].The peptides were synthesized by solid phase method by the strategy based on using of Fmoc-protective groups, diluted in sterile distilled water in concentrations from 0.25 μg/ml to 4.0 μg/ml and then were added to culture flasks for 2 or 3 days (48-72 h) followed by standard MTT-assay. Statistical significance was determined by ANOVA unpaired t test using the Prism 4.0 software (GraphPad), values of p < 0.05 were considered as significant. Each test was performed at least three times. Cationic peptid NC-811 was labeled by nontoxic Cy5 FL (Fig.1). For visualization, melanoma cells were grown on Lab-chambered coverglasses (Eppendorf) for 2 days , then 0,5 μg/ml NC-Cy was added into each chamber ,except control ones, for 2-6 h followed by fluorescent microscope analysis. Nuclei were labeled with 1 μg/ml Höchst-33342 (Khelicon ) staining to detect cell apoptosis. Labelled P53 and C23 (nucleolin) were visualized with FL GelSustain and Abcam Immunocitochem Detection system (Dia-m.ru).
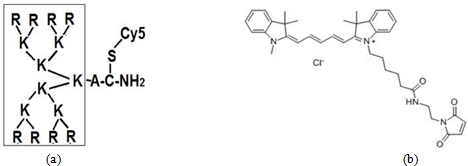
Fig. 1 – Structure of cationic peptid NC-811 (a) labelled by FL Cy5 (b)
Results
The use of cationic peptides with specific molecular structure for inducing selective tumor cell death, namely cutaneous melanoma, seems to solve a problem of melanoma resistance to standard treatment. The advantages of cationic dendritic peptides: (1) the toxicity is much less than that of linear peptides with similar amino acid composition, (2) rapid penetration through cell membranes, (3) the interaction with surface and intracellular targets tumor cells, for example, nucleolin molecules expressed on cell membrane.. Five Arg/Lys (R/K)–rich cationic peptides were selected by high antiproliferative effect after 2 or 3 days of incubation with two melanoma cell culture, while proliferation of the fibroblast cell line was normal. Main properties of these selected peptides are shown in the table 1. The inhibition of cell proliferation by other tested peptides was not effective.
Table 1 – Cationic peptides with in vitro antitumor activity for melanoma cells
| Peptid formula | Molecular mass (Daltons)/charge | Effective concentration (μg/ml) | % of surviving cells,МTT assay |
| NC-811/ R8K4K2KAC-NH2, | 2338 / 16+ | ≥0.75 | 8-10 |
| NC-798/К8K4K2KAC-NH2, | 2114 / 16+ | ≥0.25 | 25-30 |
| NC-803/(RRRKK)2KKKAC-NH2, | 2026 / 14+ | ≥0.50 | 18-20 |
| NC-780/ (K)4(K)2KAC-NH2, | 1090 / 8+ | ≥1.0 | 25-30 |
| AM2/Mir-KRPARPAR-NH2, | 1391.6 / 4+ | ≥0.50 | 30-35 |
The results of MMT assay for 4 different cationic peptides (0.25-4.0 μg/ml ) after 72h incubation with fibroblast and cutaneous melanoma cells have shown toxic effect for tumor cells compared to normal skin fibroblasts , Fig 2 (a)-(c).
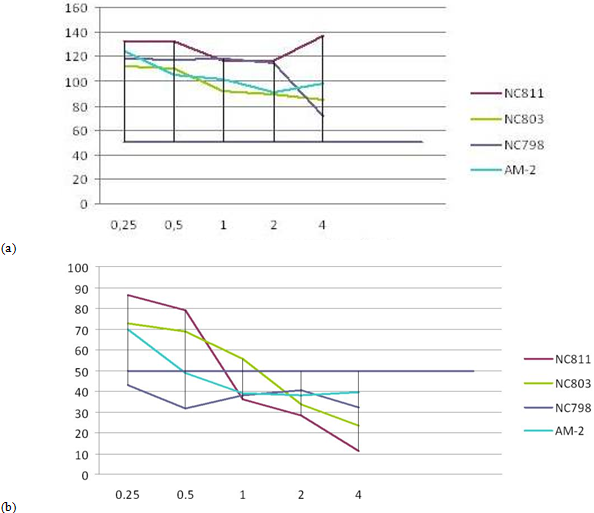
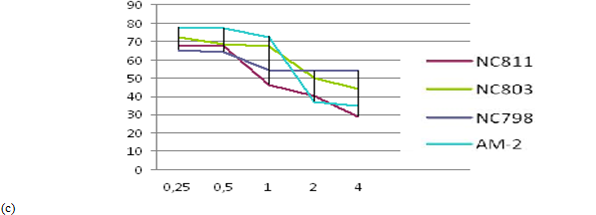
Fig. 2 ─ The results of MTT-assay for 4 cationic peptides after incubation with fibroblast cell line H1036 (a), cutaneous melanoma lines melIS (b) and melH (c). X-axis ─ peptid concentration (μg/ml); Y-axis ─ the percentage of surviving cells
In both melanoma cell lines – melIS and melH (not shown) - BRAF V600E mutations were revealed by PCR (Fig 3b). This mutation in serine-threonine kinase BRAF activates MAPK/ERK cell signallig, causes high proliferation of tumor cells and determines their sensitivity for kinase inhibitor vemurofenib (zelboraf).
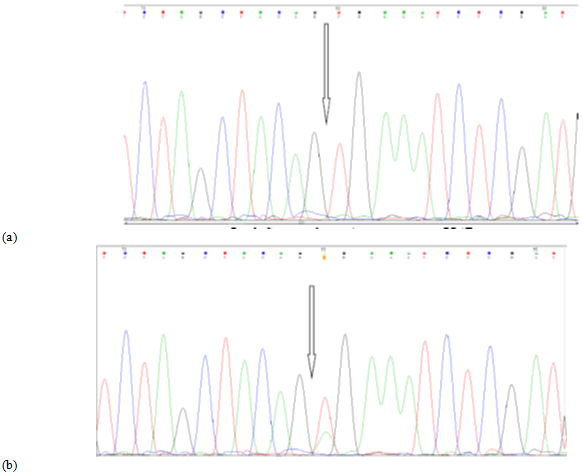
Fig. 3 ─ (a) Sanger sequence for exon 15 BRAF gene in fibroblast H1036 cell line (wt), position c.1799T is marked. (b). Sequence for exon 15 BRAF gene in melIS cell line (mut), nucleotide substitution c.1799T>A (p.V600E) is marked
The analysis of melanoma cells after incubation with fluorescent (FL)-labeled cationic peptides clarified possible role of nucleolar proteins nucleolin/ NCL and nucleophosmin/NPM in the induction of cell apoptosis (Fig 4 a-d).
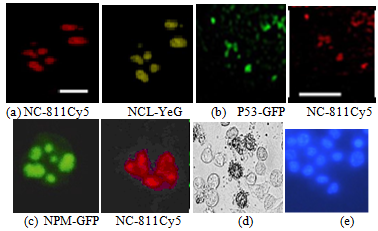
Fig. 4 – Colocalisation of FL-labeled cationic peptide NC-811Cy5 and FL-labeled NCL in melIS cell nucleus, bar = 7μk;- (a); Colocalisation of FL-labeled P53 and cationic peptide NC-811Cy5 in melH cell nucleus, bar = 25 μk – (b); Colocalisation of FL-labeled cationic peptide NC-811Cy5 and FL-labeled P53 in melIS cell nucleus; x400 – (c); tumor cell apoptosis (d); Höchst-33342 staining for melIS cells and nucleus x 1500 (e)
Discussion
Nucleolin/NCL and nucleophosmin/NPM were first discovered as major nucleolus proteins, later they were revealed to shuttle from the nucleolus to cytoplasm and the cell membrane as chaperon proteins. They are involved in many significant cell processes: regulation of transcription, translation, chromatin remodeling, ribosome biogenesis, centrosome duplication, apoptosis, cell cycle, etc. The cell surface nucleolin and nucleophosmin were shown to be associated in a nucleoprotein complex [3, P. 43685]. These proteins demonstrate a high expression level in the most solid tumors. They are involved in tumor growth, angiogenesis, and metastasis. Differential expression of NCL/NPM in normal and tumor cells (Fig. 5), might be a base for inhibition of tumor cell proliferation with low toxic effect [4, P. 127]. Here, we demonstrated that cationic peptides enriched by Arg(R)/Liz(K) with low toxicity, penetrates melanoma cells and reach the nucleus, apparently, by interaction with molecule of membrane nucleolin and then – with nucleophosmin. Preliminary Western bloting results (not shown) indicate to this mechanism as a real possibility.
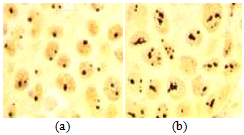
Fig. 5 – Different expression level of NCL and NPM in normal fibroblasts (a) and melanoma cells (b), AgNO3- staining of tissue sections, x 500
Cationic peptide NC-811 bearing the fluorescent label CY-5 has shown to penetrate into cells as was observed for lysine dendrimers used for anti-cancer drug delivery [5, P. 469-471]: A colocalization NC-811 with labeled /NPM/P53 in the nucleus after 2-6 hours of cell incubation with the peptide solution (1μg/ml) was found. Apoptosis as a potential cause for inhibition of cutaneous melanoma cell proliferation was revealed using Höchst-33342 staining, while normal skin fibroblasts proliferated in cultural medium with peptid supplied. According the results of MTT-assay, the highest inhibitory activity was shown for cationic peptides with dendritic structure. A possible mechanism of selective antiproliferative effect is the interaction of peptid molecule with NCL molecule overexpressed on tumor cell membrane followed by release of P53 previously associated with C23. It remains to be under study now. Understanding the mechanisms of cationic peptid action is essential for their hypothetical pharmaceutical use
Conclusion. The inhibition of cutaneous melanoma cell proliferation induced by several cationic peptides in vitro has been found. The data obtained indicate to the role of major nucleolar proteins nucleolin and nucleophosmin in this process. Further research would clarify this question.
Список литературы / References
- Fujiki H. Cell-surface nucleolin acts as a central mediator for carcinogenic, anti-carcinogenic, and disease-related ligands / H. Fujiki, T. Watanabe, M. J. Suganuma // Cancer Res Clin Oncol. – 2014. – Vol. 140 – P. 689–699.
- Хаитов М.Р. Средство для доставки нуклеиновых кислот в клетки млекопитающих / Хаитов М.Р., Чупина Н.А., Шиловский И.П. и др. // Патент RU 2572575.
- Destouches D. Multivalent pseudopeptides targeting cell surface nucleoproteins inhibit cancer cell invasion through tissue inhibitor of metalloproteinases 3 (TIMP-3) release / D. Destouches, E. Huet, M. Sader and others // J.Biol Chem. –2012. – Vol 287(52). – P. 43685-93.
- Lushnikova A. A possibility for therapy of metastatic cutaneous melanoma with cationic peptides / A. Lushnikova, D. Ponkratova, S. Andreev and others // Europ.J.Cancer. – 2017. – Vol.72. – P. 127-128.
- Zhao J. Cell-penetrable lysine dendrimers for anti-cancer drug delivery: synthesis and preliminary biological evaluation / Zhao J.,Zhou R.,Fu X. and others // Arch. Pharm. – 2014. – Vol. 347(7). – P. 469-477.
Список литературы на английском языке / References in English
- Fujiki H. Cell-surface nucleolin acts as a central mediator for carcinogenic, anti-carcinogenic, and disease-related ligands / H. Fujiki, T. Watanabe, M. J. Suganuma // Cancer Res Clin Oncol. – 2014. – Vol. 140 – P. 689–699.
- Haitov M.R. Sredstvo dlja dostavki nukleinovyh kislot v kletki mlekopitajushhih [Means for the delivery of nucleic acids to mammalian cells] / Haitov M.R., Chupina N.A., Shilovskij I.P. and others // Patent RU 2572575.
- Destouches D. Multivalent pseudopeptides targeting cell surface nucleoproteins inhibit cancer cell invasion through tissue inhibitor of metalloproteinases 3 (TIMP-3) release / D. Destouches, E. Huet, M. Sader and others // J.Biol Chem. –2012. – Vol 287(52). – P. 43685-93.
- Lushnikova A. A possibility for therapy of metastatic cutaneous melanoma with cationic peptides / A. Lushnikova, D. Ponkratova, S. Andreev and others // Europ.J.Cancer. – 2017. – Vol. 72. – P. 127–128.
- Zhao J. Cell-penetrable lysine dendrimers for anti-cancer drug delivery: synthesis and preliminary biological evaluation / Zhao J.,Zhou R.,Fu X. and others // Arch. Pharm. – 2014. – Vol. 347(7). – P. 469–477.
