Studying of ice-forming properties of aluminum oxide clusters
Studying of ice-forming properties of aluminum oxide clusters
Abstract
The article presents studies of the ice-forming properties of aluminum oxide with the aim of possibly increasing the effectiveness of pyrotechnic compositions used in anti-hail products. Increasing the efficiency of pyrotechnic compositions is achieved through experimental selection of components. First of all, by grinding and mixing the components of the pyrotechnic mixture. As a result of laboratory studies, aluminum oxide clusters were obtained in the presence of water vapor at subzero temperatures. A special set of equipment and experimental techniques have been created. It was found that the specific yield of ice-forming nuclei from aluminum oxide clusters is less than when sublimating the standard pyrotechnic composition AD-1, but at the same time the response threshold of the reagent increased to -3 ºС. Aluminum oxide clusters can be recommended for obtaining a more effective pyrotechnic composition for weather modification.
1. Introduction
The purpose of weather modification processes is to reduce the negative consequences for the economy and population from the effects of drought, reduce fire danger in forests and other natural areas, improve weather conditions over specified areas, etc. Most of these projects use pyrotechnic compound, which includes silver iodide, on the particles of which ice crystals are formed
, .Currently, due to the expanding practice of carrying out active actions with a simultaneous increase in prices for precious metals, the relevance of searching for ice-forming reagents with low and ultra-low silver content has increased. Attempts to introduce into practice the active effects of such formulations have been made before, however, since there was information that such formulations require naturally unrealizable supersaturation of water vapor, the development and implementation of such formulations was essential they are limited in this way
, .Studies of a significant number of substances have made it possible to identify a number of chemical elements that are characterized by high efficiency of ice formation and have sufficient thermal stability. Among the tested substances, zinc oxide has a high efficiency of ice formation. It has a high threshold of crystallizing action and a significant yield of crystallization nuclei compared to analogues
.2. Research methods and principles
This article presents the studies of the aluminum oxide ice-forming properties. In terms of prevalence in the earth's crust, aluminum ranks first among metals, its content is 8.8% by weight, giving way to oxygen, hydrogen and silicon. It is present only in the form of natural minerals. One of the constituent minerals is aluminum oxide with the chemical formula Al2O3. Aluminum oxide has characteristic structural features and can crystallize in various polymorphic modifications (so-called phases). The phases of Al2O3 in the literature are denoted by Greek letters. Figure 1 shows the diagram of the transition phases of aluminum oxide.
![Figure 1 - Temperature diagram of the transition modifications of Al2O3 [6]](/media/images/2024-07-26/dc9c0a2e-a38d-4fac-8a45-2b493f354f6f.jpg)
Figure 1 - Temperature diagram of the transition modifications of Al2O3 [6]
For the experiments, the following equipment was used: a cloud chamber, a device for the reagent sublimation, a fog generator, electronic scales, an optical microscope and thermostated substrates. The experiments were carried out under conditions close to full-scale, at temperatures ranging from -3 to -12oС.
When testing reagents by traditional methods, sublimation is performed in a dry chamber, then the products of the distillation are transferred to a cloudy environment. With this method of testing, metals do not exhibit their ice-forming properties, for their manifestation it is necessary that the distillation be carried out in a cloudy environment, i.e. in conditions close to real use.
During the tests, the following parameters are monitored using measuring instruments: temperature measurement in cloud and aerosol chambers should be carried out by a temperature meter with a range of measured temperatures from minus 25 to 25 oС and an error of no more than ±0.3oС; time measurement should be carried out by a stopwatch with a division price of 1 s; measurement of the mass converted into aerosol substances are produced using microanalytical scales with an accuracy of 0.01 mg.
Currently, there are no theoretical papers fully describing the process of formation of aluminum oxide clusters. Therefore, the main data in this area of knowledge are obtained as a result of experimental studies. In the patent of S.A. Novopashin and A.I. Zaikovsky
considers a possible mechanism for the formation of hollow aluminum oxide nanoparticles. This mechanism was described mathematically, and a correlation was established between the size of the initial agglomerate and the size of the resulting hollow particle. When aluminum is sublimated in water vapor in a water vapor medium at a sublimation temperature of 600-800oС aluminum interacts with water and aluminum oxide particles are formed. At temperatures of 800-1200oС aluminum is partially recovered from the oxide.All aluminum particles in contact with air are covered with a protective oxide-hydroxide film. An oxide film of this thickness can significantly reduce the rate of diffusion of the oxidizer from the reaction medium to the metal surface, which will significantly slow down the oxidation process. However, a relatively thin layer of oxide on the surface of aluminum particles is not an obstacle to the oxidation of particles by water. The process of interaction of aluminum with water is accompanied by the release of heat. During the oxidation process, the aluminum particle is completely covered with pseudobemite plates, and the shell structure of such plates is heterogeneous and loose. Due to its heterogeneity, the shell of pseudobemite nanoplates does not prevent the diffusion of water to the aluminum core and the complete transformation of the particle gradually occurs.
3. Main results
According to experimental data, the temperature of thermal sublimation of aluminum, air humidity and gas components of the medium affect the shape and size of aluminum oxide clusters. Heated aluminum actively reacts with supercooled water vapor, forming aluminum oxide clusters of various sizes. Figure 2 shows a photograph of an aluminum oxide cluster.
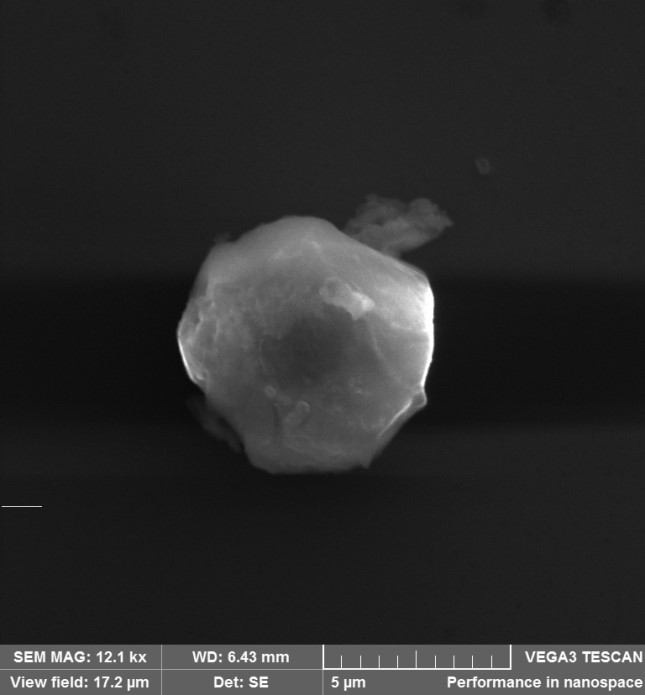
Figure 2 - Photograph of an oxide cluster appears under an electron microscope
Table 1 - Specific yield of standard pyrotechnic composition AD-1 and aluminum oxide clusters
AD-1 | Al2O3 | ||
t, оС | Specific yield, g-1 | t, оС | Specific yield, g-1 |
-11.9 | 4.4·1012 | -12 | 5.9·1011 |
-11.3 | 3.7·1012 | -12 | 4.7·1011 |
-10.6 | 3.8·1012 | -11 | 4.9·1011 |
-10.2 | 3.2·1012 | -11 | 3.7·1011 |
-9.7 | 3.6·1012 | -10 | 2.9·1011 |
-9.6 | 2.7·1012 | -10 | 5.0·1011 |
-9.5 | 3.0·1012 | -9 | 3.8·1011 |
-9.4 | 2.3·1012 | -9 | 3.4·1011 |
-9.1 | 3.2·1012 | -8 | 3.1·1011 |
-8.8 | 2.6·1012 | -8 | 4.5·1011 |
-8.5 | 2.3·1012 | -7 | 2.8·1011 |
-8.2 | 2.7·1012 | -7 | 3.5·1011 |
-7.8 | 2.8·1012 | -6 | 4.0·1011 |
-7.5 | 2.2·1012 | -6 | 3.3·1011 |
-7.3 | 2.6·1012 | -6 | 2.8·1011 |
-7 | 1.8·1012 | -5 | 2.8·1011 |
-6.8 | 1.3·1012 | -5 | 1.8·1011 |
-6.1 | 9.6·1011 | -4 | 3.1·1011 |
-5.4 | 8.2·1011 | -4 | 2.6·1011 |
– | – | -3 | 2.7·1011 |
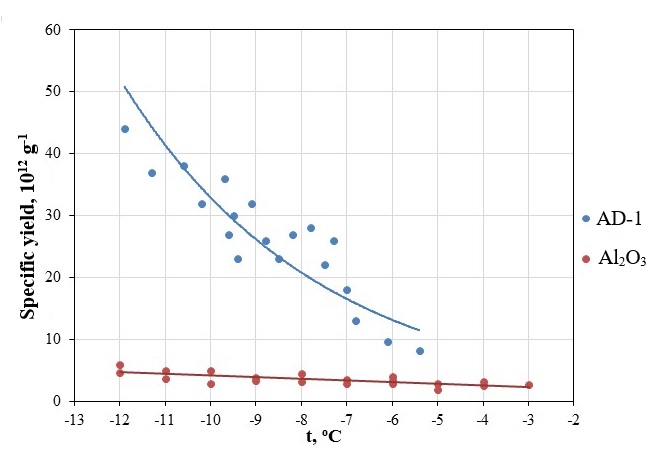
Figure 3 - Specific yield of standard pyrotechnic composition AD-1 and aluminum oxide clusters
The specific yield of ice-forming nuclei of aluminum oxide clusters ranges from 2.7×1011 to 4.7×1011 g-1 in the temperature range from -3 to -12oС. It is obvious that the specific yield of aluminum oxide clusters is less than that of the pyrotechnic composition AT-1, but at the same time the threshold for reactant activation has increased to -3oС. This result is explained that the nanofibers that make up the aluminum oxide clusters are closed and the effect of forming an ice structure is not observed in them.
The principal possibility of using aluminum is due to the rapid growth of ice crystals on aluminum oxide particles.
The parameter, like the speed of the reagent, is estimated by the number of active ice-forming nuclei within 2 minutes after the reagent sublimation
.Figure 4 shows the results of studying the activation rate of aerosols formed using the most well-known pyrochemicals
. For comparison, we have added the results of our study of the rate of nucleation of the reagent from aluminum oxide clusters.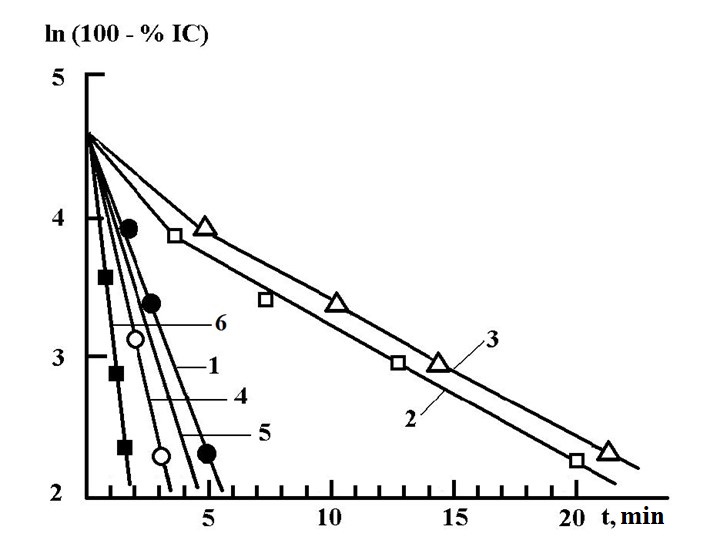
Figure 4 - Nucleation rate of ice-forming compounds
Note: Note: 1 – BR-91-Y; 2 – 50-04-112- measurements taken in China; 3 – NEI-TB1; 4 – 50-04-11 - measurements were performed at the Central Aerological Observatory (Russia); 5 – 50-04-11 - measurements performed by Feder (Switzerland); 6 – aluminum oxide clusters - measurements were performed by us
According to experimental data, the tested reagent can be used to reduce "unproductive" losses of cloud moisture and increase the efficiency of precipitation formation.
As is known, only part of the water of clouds during natural processes is realized in the form of precipitation. R. Braham (1952) states that during thunderstorms, only 19% of cloud moisture falls in the form of rain. In the work of P. Vukoff (1996), only 10% of the total mass of liquid water contained in it falls from a convective cloud, and only 25-30% of moisture falls from orographic clouds . R. Eliot (1960) found that 50-70% of the moisture contained in them falls in the form of precipitation from cumulus clouds .
4. Conclusion
Aluminum oxide clusters have ice-forming properties with a crystallization threshold of -3C. They can be used to develop a more effective pyrotechnic composition and its introduction into the composition of rockets designed to stimulate earlier and faster (than in the natural course of processes) precipitation formation in areas of future hailstorm. Such an impact will lead to premature precipitation, erosion of water content in areas of future hail formation, aerodynamic braking, suppression of updrafts and elimination of conditions for the formation of hail particles
.It should be noted that natural disasters are an integral part of society's life, and as it develops, the danger of exposure to natural processes and the scale of human and material losses associated with them increase. On average, 5 hail storms of catastrophic intensity are observed in the territory of the North Caucasus Federal District per year, the average annual damage from which is about 11% of all natural emergencies.
Note that the widespread occurrence of aluminum in nature and its cheapness compared to silver, by more than 2 orders of magnitude, play an important role. Thus, the use of aluminum will significantly reduce the cost of the reagent and the impact technology in general.
