ВЛИЯНИЕ ПРИРОДЫ НЕНУКЛЕОТИДНЫХ ВСТАВОК НА ТЕРМОСТАБИЛЬНОСТЬ G-КВАДРУПЛЕКСОВ ДНК
Секридова А.В.1, Варижук А.М.2, Позмогова Г.Е.3
1ORCID: 0000-0002-0440-4673, аспирант, 2ORCID: 0000-0001-9359-8651, Кандидат химических наук, 3ORCID: 0000-0001-9458-4972, Доктор химических наук, профессор, ФГБУ ФНКЦ Физико-Химической Медицины ФМБА России
Работа выполнена при поддержке гранта РНФ №14-25-00013
ВЛИЯНИЕ ПРИРОДЫ НЕНУКЛЕОТИДНЫХ ВСТАВОК НА ТЕРМОСТАБИЛЬНОСТЬ G-КВАДРУПЛЕКСОВ ДНК
Аннотация
Известно, что в человеческом геноме каноническая двойная спираль сосуществует с G-квадруплексными структурами, обладающими важными биологическими функциями. Исследования последних лет продемонстрировали взаимосвязь G-квадруплексов (G4) cо многими человеческими заболеваниями и указали перспективное направление для создания лекарственных препаратов и диагностикумов. В данной статье показано, что введение в петли природных параллельных G4 - фрагментов Alu-повторов и микросателлитов - производных триэтиленгликоля дестабилизировало структуру, а тетрагидрофурана значительно повышало ее термостабильность при сохранении нативной топологии G4. Найденные закономерности важны для исследования конформационного полиморфизма полинуклеотидов, а предложенные химические модификации – для создания диагностикумов и лекарств на основе G4.
Ключевые слова: G-квадруплекс ДНК, синтез модифицированных олигонуклеотидов, конформационный полиморфизм полинуклеотидов
Sekridova A.V.1, Varizhuk A.M2, Pozmogova G.E3
1ORCID: 0000-0002-0440-4673, Postgraduate stuent, 2ORCID: 0000-0001-9359-8651, PhD in Chemistry, 3ORCID: 0000-0001-9458-4972, PhD in Chemistry, professor, Federal Research and Clinical Center of Physical-Chemical Medicine of Federal Medical & Biological Agency
This work was supported by RSF №14-25-00013
SOCIAL INSURANCE IN THE RUSSIAN FEDERATION THE IMPACT OF NON-NUCLEOTIDE INSERTIONS ON THERMAL STABILITY OF DNA G-QUADRUPLEXES
Abstract
The canonical double helix in human genome coexists with G-quadruplex structures that are known to have important biological functions. Recent research has established clear connections between G-quadruplexes (G4) and various human diseases, which opens up new possibilities for targeted drug therapies and diagnosticums. We show here that replacing single-nucleotide loops in parallel genomic G4 - fragments of Alu repeats and microsatellites - with triethylene glycol moieties destabilized G4 structures, while introducing tetrahidrufuran derivatives into the loops, alternatively, resulted in significant stabilization. The non-nucleotide insertions in the loops did not alter G4 topologies. Our findings provide important insight into conformational polymorphism of polynucleotides. The chemical modifications discussed in this paper may be of use for developing G4-based oligonucleotide therapeutics.
Keywords: G-quadruplex DNA, synthesis of modified oligonucleotides, conformational polymorphism of polynucleotides
The formation of non-canonical DNA structures (ncDNA) is currently regarded as an important element of gene expression regulation, and specific G4-protein interactions are known to underlie the development of many pathological states (e.g., с-Myc superexpression, Werner and Blum syndromes, neurodegenerative diseases, etc.). Baral et al. have demonstrated the relation between the proteomic profile changes and SNPs that affect G4 conformations in over 50 mutant sites [1]. The development of G4-targeting anticancer agents and other therapeutics is a promising trend in pharmacology.
G4 (G-quadruplex) is a G-rich polynucleotide fragment that is folded into a four-stranded helical structure composed of stacked G-tetrads. Each G-tetrad is a planar arrangement of four guanines stabilized by eight H-bonds. Pi-pi stacking of the tetrads imparts additional stability to the structure.G4 structures are highly diverse, with different relative strand orientations, number of tetrads, loop lengths and sequences, etc. Basic G4 topologies are classified into parallel (all four stands have similar orientations), antiparallel and hybrid.
Intramolecular parallel G4s attract much attention due to their putative biological role. Sequences that may adopt such structures are rather abundant in the human genome and are typically found in oncogen promoter regions. Therefore, they are regarded as attractive therapeutic targets [2]. What is more, these structures constitute the core of many potent aptamers to human and viral proteins [3-4].
Local chemical modification of synthetic oligonucleotides is one of the most promising approaches to creating effective G4 drugs [5-12].
Previously, we have shown that modifications of the oligonucleotide backbone, which do not affect G-tetrads (for example, phosphorothioate internucleotide bonds), are typically compatible with the required folding of G4-DNA aptamers [10, 12].
In this study, we focus on identifying thermodynamically favorable modifications of 2/3-tetrad parallel G4s with single-nucleotide loops – structural motifs that are widely represented in Alu-repeats and microsatellites of the human genome [4, 13-14]. G4s with single-nucleotide loops are convenient models for investigating the effects of loop modifications. Putative G4s with single-nucleotide loops are also the most frequent in the human genome. Short loop length and high thermal stability determine genomic instability induced by G-quadruplex-forming minisatellites.
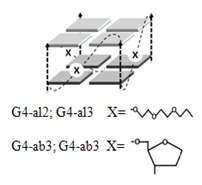
Based on the analysis of experimental and literature data and the use of molecular modeling techniques [15], we proposed the following strategy for designing potentially highly thermostable G4 structures: single-nucleotide loops of known 2/3-tetrad G4s (G4-3/G4-2) are replaced with non-nucleotide (triethylene glycol or tetrahydrofuran) fragments, fig.1. Thiethylene glycol linkers were chosen because of their relative flexibility and adequate length (close to that of a single nucleotide loop). Tetrahydrofuran-based linkers imitate, to some extent, abasic sites in oligonucleotides. Because the exposure of hydrophobic nucleobases to hydrophilic environment (solution) is thermodynamically unfavorable, we assumed that the absence of heterocyclic bases in loops may facilitate G4 folding. What is more, non-natural fragments in loops are likely to substantially improve oligonucleotide resistance to enzymatic hydrolysis, considering that loops are usually the predominant positions of nuclease attacks [10, 12].
Sequences of the oligonucleotides are presented in Table 1. The oligomers were synthesized by the solid-phase method (ASM-800 synthesizer, Biosset, RF, amidites - GlenRes, USA); purified by HPLC (purity> 97%) and characterized as described in [16].
Prior to the spectrophotometric measurements, the oligonucleotides were dissolved in a 20 mM sodium phosphate buffer containing 100 mM KCl (pH 7.5). The oligonucleotide single strand concentrations were calculated from the absorbance measured above 80°C and the extinction coefficients, which were approximated using the nearest-neighbor model. The samples were denatured at 95°C for 5 min and cooled quickly to 15°C prior to measurements. The UV melting curves were recorded on a spectrophotometer equipped with a thermostated cuvette holder. The absorbance was registered at λ = 295 nm every 0.5°C across the 15–90°C temperature range. The melting temperatures of the quadruplexes were defined by performing a fitting procedure using the two-state model for monomolecular melting in KaleidaGraph version 4.0.
Melting temperatures of G4-3, G4-2 and their analogs are presented in Table 1. CD spectra, UV-melting curves, thermal difference spectra (TDS) and the analysis of rotation relaxation times (RRT) of EtBr fluorescence in complexes with G4s (which are roughly proportional to the hydrodynamic volume of the complexes) confirm monomolecular G4 folding of these ONs at low/medium concentration.
Figure 1. Schematic representation of the modified G4s.
Table 1. Oligonucleotide sequences and thermal stabilities of G4s.
| ON Code | Sequence, 5’- | Tm±sd, °C, (mM KCl) |
| G4-2 | G2AG2CG2AG2 | 74±1(100) |
| G4-al2 | [G2teg]3G2 | 50±2 (100) |
| G4-ab2 | (G2tgf)3G2 | 85±1 (100) |
| G4-3 | (G3T)3G3 | >90 (100) |
| >85(10) | ||
| 44±2 (100 mM LiCl) | ||
| G4-al3 | [G3teg]3G3 | >90 (100) |
| G4-ab3 | (G3tgf)3G3 | >90 (100) |
Positive peaks at ~265 nm in the CD spectra suggest parallel G4 folding of all ONs. The G4-3 series ONs are stable in the presence of KCl even at 90°C and retain G4 folding at RT in the presence of LiCl. The alcoxy (triethylene glycol-based) loop modification decreases G4 thermal stability (by 24±2°C in the G4-2 series), whereas introduction of abasic sites (the tetrahydrofuran-based loop modification) leads to certain stabilization.
NMR data confirm that the modifications do not cause any principal alterations in the G4 topologies. Twelve signals in the imino-proton regions of G4-ab3 1H-spectra are consistent with 3-quartet structures. Twelve signals in the imino-proton regions of G4-ab3 1H-spectra NMR are consistent with 3-quartet structures. Eight signals in the case of G4-ab2 agree with the 2-quartet structure.
In conclusion, we have shown that introducing triethyleneglycol moieties into loops of parallel G4s decreased thermal stabilities of the structures, whereas the tetrahydrofuran-based modification resulted in enhanced thermal stability. Importantly, the parallel topology of G4s was maintained. The impact of the tetrahydrofuran-based modification in loops on the stabilities of other G4 structures (G4 aptamers in particular) will hopefully be the subject of future studies. Replacing G4 loops or loop fragments with tetrahydrofuran moieties and monitoring changes in G4 activity/binding affinity could be useful for identifying binding epitopes or clarifying the role of loop sequences.
To summarize, our findings provide important insight into conformational polymorphism of polynucleotides. The new G4s with remarkably high thermal stabilities may be of use for developing oligonucleotide therapeutics and diagnosticums.
References
- Baral A., Kumar P., Halder R., Mani P., Yadav V.K., Singh A., Das S.K.Chowdhury S. Quadruplex-single nucleotide polymorphisms (Quad-SNP) influence gene expression difference among individuals // Nucleic Acids Res. - 2012; 40 (9): 3800-3811.
- Tsvetkov V., Pozmogova G.Varizhuk A. The Systematic Approach to Describing Conformational Rearrangements in G-quadruplexes // J Biomol Struct Dyn. - 2015; 1-36.
- Varizhuk A., Ilyinsky N., Smirnov I.Pozmogova G. G4 Aptamers: Trends in Structural Design // Mini Rev Med Chem. - 2016.
- Severov S., Varizhuk A., Smirnov I.Pozmogova G. Investigation of the G4 interactome using human protein microarrays // Febs Journal. - 2015; 282 56-56.
- Tsvetkov V.B., Varizhuk A.M., Pozmogova G.E., Smirnov I.P., Kolganova N.A.Timofeev E.N. A Universal Base in a Specific Role: Tuning up a Thrombin Aptamer with 5-Nitroindole // Scientific Reports. - 2015; 5.
- Tatarinova O., Tsvetkov V., Basmanov D., Barinov N., Smirnov I., Timofeev E., Kaluzhny D., Chuvilin A., Klinov D., Varizhuk A.Pozmogova G. Comparison of the 'Chemical' and 'Structural' Approaches to the Optimization of the Thrombin-Binding Aptamer // PLoS ONE. - 2014; 9 (2).
- Kolganova N.A., Varizhuk A.M., Novikov R.A., Florentiev V.L., Pozmogova G.E., Borisova O.F., Shchyolkina A.K., Smirnov I.P., Kaluzhny D.N.Timofeev E.N. Anomeric DNA quadruplexes: Modified thrombin aptamers // Artif DNA PNA XNA. - 2014; 5 (1).
- Varizhuk A.M., Tsvetkov V.B., Tatarinova O.N., Kaluzhny D.N., Florentiev V.L., Timofeev E.N., Shchyolkina A.K., Borisova O.F., Smirnov I.P., Grokhovsky S.L., Aseychev A.V.Pozmogova G.E. Synthesis, characterization and in vitro activity of thrombin-binding DNA aptamers with triazole internucleotide linkages // European Journal of Medicinal Chemistry. - 2013; 67 90-97.
- Zaitseva M., Kaluzhny D., Shchyolkina A., Borisova O., Smirnov I.Pozmogova G. Conformation and thermostability of oligonucleotide d(GGTTGGTGTGGTTGG) containing thiophosphoryl internucleotide bonds at different positions // Biophysical Chemistry. - 2010; 146 (1): 1-6.
- Pozmogova G.E., Zaitseva M.A., Smirnov I.P., Shvachko A.G., Murina M.A.Sergeenko V.I. Anticoagulant Effects of Thioanalogs of Thrombin-Binding DNA-Aptamer and Their Stability in the Plasma // Bulletin of Experimental Biology and Medicine. - 2010; 150 (2): 180-184.
- Luk'ianova T.A., Zaiiitseva M.A., Karpov V.A.Pozmogova G.E. [The synthesis and mass spectrometry of oligonucleotides bearing thiophosphoryl modifications of the predetermined localization] // Bioorg Khim. - 2008; 34 (1): 83-88.
- Zaitseva M., Kaluzhny D., Shchyolkina A., Borisova O., Smirnov I.Pozmogova G. Conformation and thermostability of oligonucleotide d(GGTTGGTGTGGTTGG) containing thiophosphoryl internucleotide bonds at different positions // Biophys Chem. - 2010; 146 (1): 1-6.
- Pozmogova G., Severov V., Varizhuk A., Smirnov I., Sekridova A.Tsvetkov V. Secondary DNA structures in G/C-rich microsatellites. Investigating the equilibrium between G-quadruplexes, I-motifs and duplexes // Febs Journal. - 2014; 281 639-640.
- Bose P., Hermetz K.E., Conneely K.N.Rudd M.K. Tandem repeats and G-rich sequences are enriched at human CNV breakpoints // PLoS ONE. - 2014; 9 (7): e101607.
- Tsvetkov V., Pozmogova G.Varizhuk A. The systematic approach to describing conformational rearrangements in G-quadruplexes // J Biomol Struct Dyn. - 2016; 34 (4): 705-715.
- Abu-Ghazalah R.M., Irizar J., Helmy A.S.Macgregor R.B., Jr. A study of the interactions that stabilize DNA frayed wires // Biophys Chem. - 2013; 147 (3): 123-129.