ИССЛЕДОВАНИЕ ФУНКЦИОНАЛИЗИРОВАННЫХ ОКСИДА ГРАФЕНА И ДИВИНИЛЬНОГО КАУЧУКА, СОДЕРЖАЩЕГО ОКСИД ГРАФЕНА, МЕТОДАМИ ИК-СПЕКТРОСКОПИИ И ТЕРМОГРАВИМЕТРИЧЕСКОГО АНАЛИЗА
Набиев А.М. 1, Алиев Э.М. 2, Азизов А.А. 3, Алосманов Р.М. 4, Ейвазова Г.М. 5, Меликова А.Я. 6, Буният-Заде И.А. 7
1,2 Аспирант; 3,4 Профессор, Доктор химических наук; 5 Кандидат физических наук; 6,7 Кандидат химических наук , 1-5, 7 Бакинский Государственный Университет, 6 Азербайджанская Государственная Нефтяная Академия
ИССЛЕДОВАНИЕ ФУНКЦИОНАЛИЗИРОВАННЫХ ОКСИДА ГРАФЕНА И ДИВИНИЛЬНОГО КАУЧУКА, СОДЕРЖАЩЕГО ОКСИД ГРАФЕНА, МЕТОДАМИ ИК-СПЕКТРОСКОПИИ И ТЕРМОГРАВИМЕТРИЧЕСКОГО АНАЛИЗА
Аннотация
В данной работе представлены результаты исследования оксида графена и дивинильного каучука, содержащего оксид графена, функционализированных –PO(OH)2 кислотными группами в результате реакции окислительного хлорфосфорилирования под действием PCl3 в присутствии кислорода. Функционализация подтверждена методами ИК спектроскопии и термогравиметрического анализа.
Ключевые слова: оксид графена, дивинильный каучук, функционализация, ИК спектроскопия, ТГ анализ.Nabiyev A.M. 1, Aliyev E.M. 2, Azizov A.A. 3, Alosmanov R.M 4 , Eyvazova G.M.5, Melikova A.Y. 6, Buniyad-Zadeh I.A. 7
1,2 postgraduate student; 3,4professor, PhD in Chemistry; 5PhD in Physics; 6,7PhD in Chemistry, 1-5, 7Baku State University, 6Azerbaijan State Oil Academy
IR SPECTROSCOPY AND TG ANALYSIS INVESTIGATIONS OF FUNCTIONALIZED GO AND GO-CONTAINING DIVINYL RUBBER
Abstract
Graphene oxide (GO) sheets and GO-containing divinyl rubber are functionalized with –PO(OH)2 groups via the reaction of oxidative chlorophosphorylation by PCl3 in the presence of oxygen. The functionalization is confirmed by Fourier transform infrared spectroscopy and TG analysis.
Keywords: graphene oxide, divinyl rubber, functionalization, IR spectroscopy and TG analysis.
Carbon is the fascinating material for science and technology. Diamond, graphite, carbyne, fullerene as the allotropic modifications are the most studied compounds. Since 1996 after a new direction in carbon chemistry has been determined, a new area has begun: the chemistry of fullerenes, carbon nanotubes and graphene.
Graphene-oxide despite of graphene single layers is an electrical insulating compound. According to the most recent studies, graphene-oxide possesses a wide range of oxygen functionalities, such as epoxide and alcohol groups on the basal planes, in addition to carbonyl, ketone, and carboxyl groups at the edges [1]. Such reactions can cause an addition of many functional groups, as, for example, of carboxyl, alcohols, ketones, or esters. (-COOH, -OH, >C=O, or –COO-, resp.) to the external walls of tubes. It should be highlighted that hydroxyl or carboxyl groups are often used as precursors for further reactions of silanisation, esterification, or alkylation and arylation [2,3].
Fig 1. The main structure of graphene-oxide
In this paper, modification of GO and GO- containing divinyl rubber via the reaction of oxidative chlorophosphorylation has been realized for improving the thermal stability properties of GO and preparation new GO-containing composite material. Oxidative chlorophosphorylation reaction is very promising as allow to enter into the structure of graphene oxide, a more thermally stable groups [4]. It is believed that thermogravimetric analysis of functionalized GO will be useful for developing methods to produce new nanocomposites by using –PO(OH)2 groups functionalized GO as fillers.
Graphite powder was used as the starting material for the synthesis of GO. The graphite had a purity of 99%. All other chemicals, such as conc. sulfuric acid (96%), chloroform, phosphorus trichloride, potassium permanganate, sodium nitrate, hydrogen peroxide (30%) were obtained from Vecton Co. (Russia) and were of reagent grade.
GO was synthesized from graphite powder using Hummers method [5]. In brief, 1 g of graphite and 0.5 g of sodium nitrate were mixed, and the mixture added to the 23 mL of concentrated sulfuric acid under constant stirring. After 15 minutes 3 g of KMnO4 was added gradually to the solution that were placed in an ice bath for preventing the temperature of the suspension from exceeding 20 °C. The mixture was stirred at 35°C for 100 minutes and the resulting paste was brownish grey in color. Then 46 mL of distilled water was added to the paste, causing violent effervescence and an increase in temperature to 98 °C. The diluted brown color suspension was maintained at this temperature for 50 minutes. Then the suspension was diluted by 250 mL of distilled water, and treated with 30 % H2O2 solution (100 ml). In a typical reaction, GO (0.5 g) was maintained into a triple-neck, round-bottom flask that was provided with reverse refrigerator, thermometer and bubbler to supply oxygen. Chloroform (0.125 mol) was added to the GO powder and stirred by oxygen flow (8 L/h). Phosphorus trichloride (0.05 mol) was gradually added into the flask for about 45 min. The reaction mixture was stirred for 3 hours at room temperature. The final product was washed with distilled water (up to pH 7).
Functionalized GO-containing polymer composite was prepared as followed. At first, GO-containing polymer suspension was prepared. For that, GO (0.25 g) was added to the solution of divinyl rubber (0.5 g polymer was dissolved 0.25 mol chloroform) and then all procedures were realized as indicated above. As a result, functionalized GO-containing divinyl rubber was obtained.
Functionalized GO and functionalized GO-containing divinyl rubber were characterized by Fourier transform infrared (FT-IR) spectroscopy (FT-IR spectrophotometer, Varian 3600).
Thermogravimetric analysis (TGA) was carried out using a STA 449 F3 Jupiter thermal analyzer (NETZSCH, Germany) with a synthetic air flow rate of 35 mL min-1 and a heating rate of 10 K min-1. The samples weights were 4 mg. They were heated from room temperature to 825 °C.
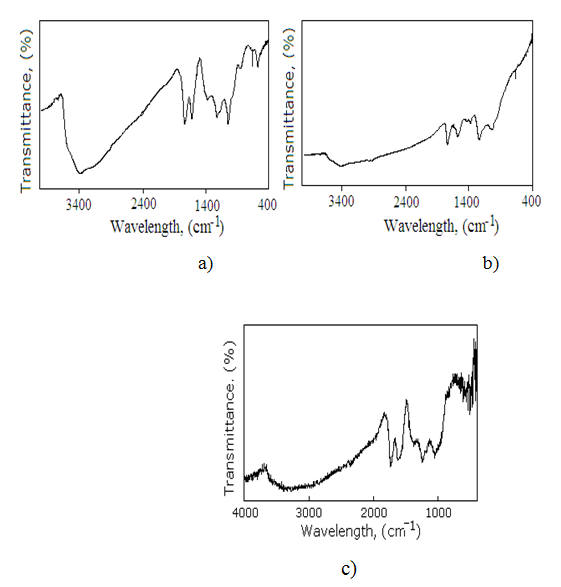
FT-IR spectra of GO, functionalized GO (FGO) and functionalized GO-containing divinyl rubber (FGO-DVR) are shown in Fig. 2. The broad band at 3385 cm-1 of GO belongs to the ν(-OH) vibration and the band at 1618 cm-1 shows that the C=C bond still remained before and after the oxidation process. ν(C=O) at 1735 cm-1 is assigned to the carbonyl and carboxyl moieties, and the vibration at 1049 cm -1 is assigned to ν(C-O) band of the epoxy group. At 1224 cm-1 observed C-OH stretching vibrations. In contrast, the stretch vibrations of aromatic ring is shown at 1576 cm -1. Transmittance bands at 1374 cm-1 and 3566 cm-1 belong to Ph-OH bend and stretch vibrations, respectively. The strong transmittance band at 1035 cm-1 is characterized to the stretch vibrations of C-O-P group. The stretch vibration for P=O is observed at 1232 cm-1.
Fig. 2. FTIR spectrums of GO (a), FGO (b) and FGO-DVR (c)
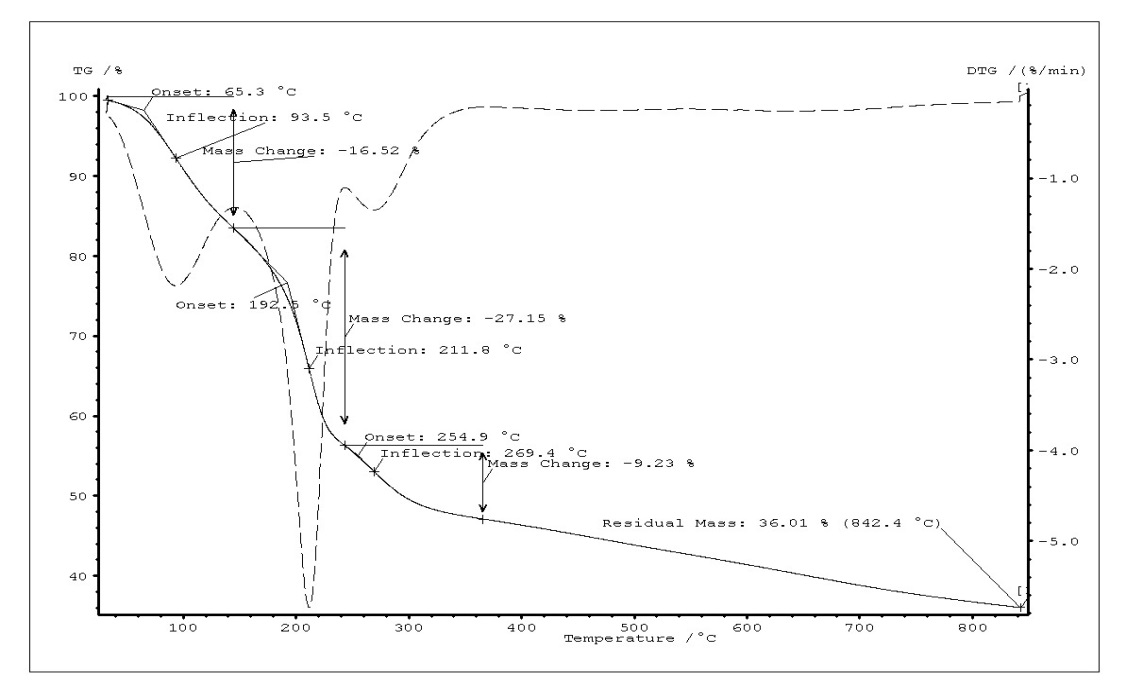
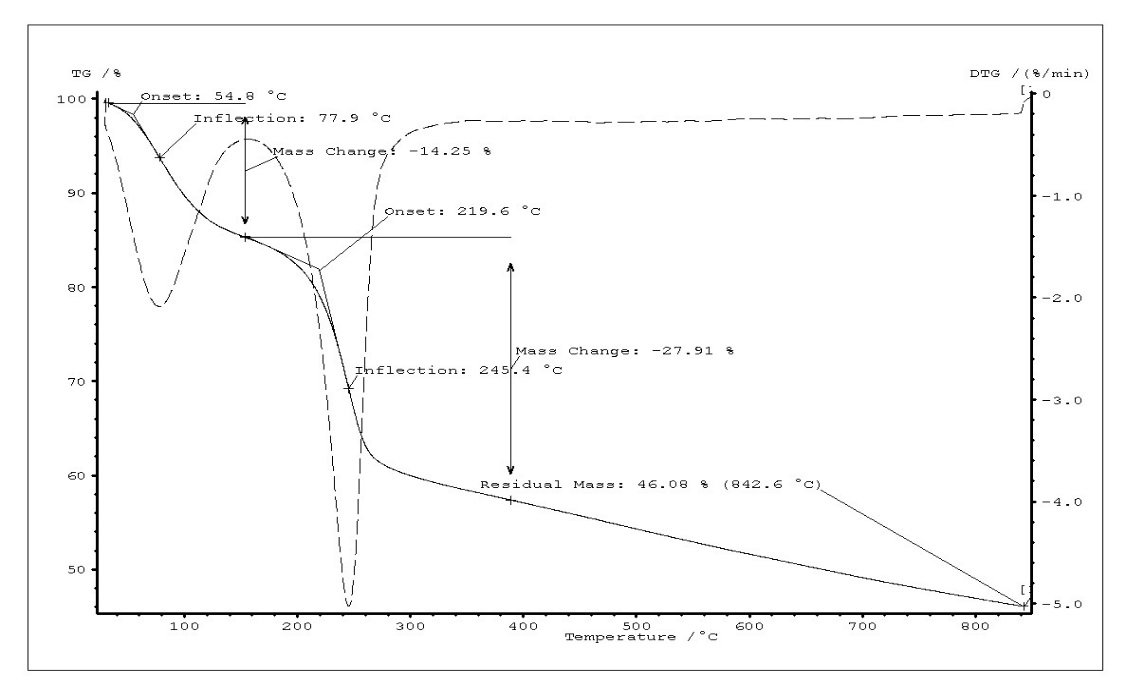
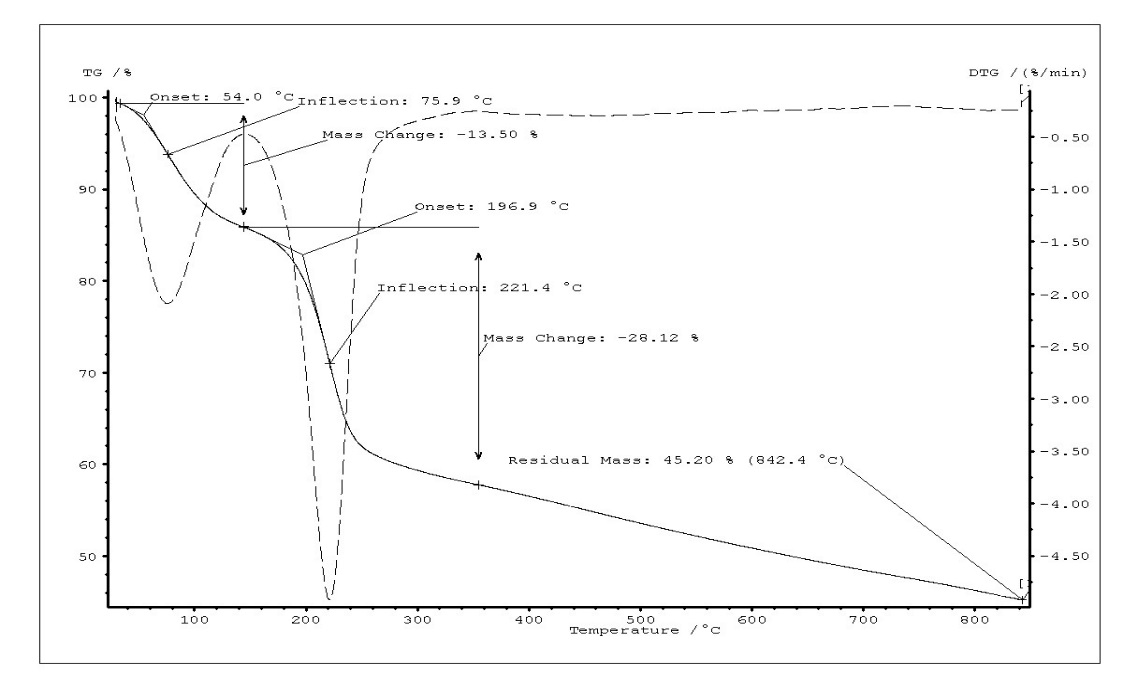
Thermogravimetric analysis of GO, FGO and FGO-DVR has been presented in Fig.3. The curves show weight profiles of powder samples as variation of temperature. It can be clearly seen that the TG curves of GO and functionalized GO showed three and two distinct steps, respectively. Weight loss (16%) of GO up to 145 °C could be primarily due to evaporation of water molecules held in the sample (Tinfl.=93.5 °C). This result is accompanied in TG curve of functionalized GO with weight loss (14 %) up to 154 °C. In the second step weight loss of both GO and functionalized GO approximately are of water, HCl, H3PO2, H3PO3 and removal of labil oxygen groups (carboxyl, anhydride, or lactone groups). (Tinfl.(GO)= 212°C, Tinfl. (Func.GO)=245°C). In this step evaporation of water could be explained interaction of neighbor OH groups in –PO(OH)2 and alcohol groups on the basal planes of the GO. A comparatively small amount (9 %) of weight loss by GO in the third step (243°C-366°C) indicates that in this step the weight loss is attributed to the removal of more stable oxygen functionalities (such as phenol, carbonyl, quinone). In contrast, functionalized GO did not show weight loss in the third step. The residual mass of GO and functionalized GO are 36% and 46%, respectively. Graphene oxide (GO) sheets is functionalized with –PO(OH)2 groups via the reaction of oxidative chlorophosphorylation by PCl3 in the presence of oxygen. It has been determined that the thermal stability of GO increased as the result substitution of hydroxyl groups on the basal planes by –PO(OH)2 groups. The oxidative chlorophosphorylation used for GO may be efficient for elaborating the polymer composites with improved thermal stability. For this reason, GO-containing divinyl rubber has been functionalized and it was detected that thermal stability of functionalized GO-containing divinyl rubber is higher than that of functionalized divinyl rubber without GO.
a)
b)
c)
Fig. 3. TG curves of GO (a), FGO (b) and FGO-DVR
References
- Cai W, Piner RD, Stadermann FJ, Park S, Shaibat MA, Ishii Y, et al. Synthesis and solid-state NMR structural characterization of 13C-labeled graphite oxide //Science. – 2008. - № 321.- Р.1815-1817.
- L. Qu, K.M. Lee, and L. Dai, Functionalized and Applications of Carbon Nanotubes. Carbon Nanotechnology. // Elsevier .- 2006. - № 133. - P. 155-234
- Z. Konya, I. Vesselenyi, K. Niesz et al., “Large scale production of short functionalized carbon nanotubes” // Chemical Physics Letters. - 2002. - № 5-6. -Vol.360. – P. 429-435.
- Akhmedov V., Sulaiman Alfadul, Maharramov A., Azizov A., Alosmanov R., Buniyad-Zadeh I., Modification of industrial divinyl rubber by oxidative chlorophosphorylation and assess-ment of metal ion removal efficiency of obtained polymer sorbent // Polish journal of Chemical Technology. – 2015. - Vol.17. - № 2. - Р. 112-118.
- Ramanathan T, Abdala AA, Stankovich S, Dikin DA, Herrera Alonso M, Piner RD, et al. Functionalized grapheme sheets for polymer nanocomposites // Nat Nano. – 2008. - № 3. - Р. 327-331.